UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_______________________________________
FORM 10-K
_______________________________________
(Mark One)
☒ | | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20202023
orOR
☐ | | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _________________ to _____ .____________
Commission File Number: 001-37844
_______________________
Bioventus Inc.BIOVENTUS INC.
(Exact nameName of registrantRegistrant as specifiedSpecified in its charter)
_______________________Its Charter)
| | | | | | | | |
| Delaware | | | | | | | | 81-0980861 |
Delaware
(State or other jurisdictionOther Jurisdiction of incorporation Incorporation or organization)
Organization) | | 81-0980861
(I.R.S. Employer Identification No.) |
| | |
| 4721 Emperor Boulevard, Suite 100 | | |
| Durham, North Carolina | | 27703 |
(Address of principal executive offices)Principal Executive Offices) | | 27703
(Zip Code) |
(919) 474-6700
(Registrant’s telephone number, including area code)
_______________________Telephone Number, Including Area Code
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Class A Common Stock, $0.001 par value $0.001 per share | BVS | The Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
_______________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No☒No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☐☒ No ☒☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T
(§ (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | ¨☐ | | Accelerated filer | ¨☒ |
| | | |
| Non-accelerated filer | ý☐ | | Smaller reporting company | ¨☒ |
| | | Emerging Growth Company | | | |
| | Emerging growth company | ý☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B)13(a) of the SecuritiesExchange Act. ý☒
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ý☒
As of June 26, 2020,July 1, 2023, the last business dayend of the registrant's most recently completed second fiscal quarter, there was no public market for the registrant's common equity and, therefore, the registrant cannot calculate the aggregate market value of its voting and non-votingClass A common equitystock held by non-affiliates as(based upon the closing price of such date.these shares on the Nasdaq) was approximately $75.4 million.
As of March 22, 2021,February 27, 2024, there were 41,038,58963,378,803 shares of Class A common stock outstanding and 15,786,737 shares of Class B common stock outstanding.
BIOVENTUS INC.DOCUMENTS INCORPORATED BY REFERENCE
TABLE OF CONTENTSCertain of the information required to be furnished pursuant to Part III of this Annual Report on Form 10-K will be set forth in, and incorporated by reference from, the registrant’s definitive proxy statement for the 2024 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission no later than 120 days after the end of the fiscal year ended December 31, 2023.
| | | | | | | | |
| BIOVENTUS INC. | |
| | |
| TABLE OF CONTENTS | |
| | |
| | Page |
| |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| | |
| | |
| | |
| | |
| | |
| |
| |
| |
| |
| |
| |
| |
| | |
| | |
| | |
| | |
| |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| | |
| | |
| | |
| |
FORWARD-LOOKING STATEMENTSTRADEMARKS, TRADE NAMES AND SERVICE MARKS
This Annual Report on Form 10-K (Annual Report)(“Annual Report”) includes our trademarks and trade names that we own or license, and our logos. This Annual Report also includes trademarks, trade names and service marks that are the property of other organizations. Solely for convenience, trademarks and trade names referred to in this Annual Report appear without any “™” or “®” symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these trademarks, trade names and service marks. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (Exchange Act)(“Exchange Act”), and Section 27A of the Securities Act of 1933, as amended (Securities Act)(“Securities Act”), concerning our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our business operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements including, without limitation, statements regarding our business strategy, including, without limitation,expectations relating to our integration of Misonix and Bioness, potential acquisitions, and expected expansion of our pipeline and research and development investment, cost savings initiatives, new therapy launches, expected timelines for clinical trial results and other development milestones, expected contractual obligations and capital expenditures, our operations and expected financial performance and condition, and impacts of the COVID-19 pandemic.condition. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words.
Forward-looking statements are based on management’s current expectations, estimates, forecasts and projections about our business and the industry in which we operate, and management’s beliefs and assumptions are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Annual Report may turn out to be inaccurate. Furthermore, if the forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. FactorsImportant factors that may cause actual results to differ materially from current expectations include, among other things, those described in Part I, Item 1A. Risk factors.Factors, which are summarized in the list below. You are urged to consider these factors carefully in evaluating these forward-looking statements. These forward-looking statements speak only as of the date hereof. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
This Annual Report includes our trademarks and trade names that we own or license, such as Bioventus, Cellxtract, Durolane, Exogen, Exponent, GELSYN-3, MOTYS, OsteoAMP, Prohesion, PureBone, SAFHS, Signafuse, SUPARTZ FX and our logo. This Annual Report also includes trademarks, trade names and service marks that are the property of other organizations. Solely for convenience, trademarks and trade names referred to in this Annual Report appear without any “™” or “®” symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these trademarks, trade names and service marks. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
SUMMARY OF PRINCIPAL RISK FACTORS
Summary of Principal Risk Factors
We are subject to several risks, including risks that may prevent us from achieving our business objectives or that may adversely affect our business, results of operations, financial condition, and cash flows. You should carefully consider the risks discussed in the section entitled Part I, Item 1A. Risk Factors, including the following principal risks:
•if we are unable to meet our current operating projections or secure other sources of liquidity, substantial doubt about our ability to continue as a going concern may arise, which may negatively affect the market value of our Class A common stock;
•our Amended 2019 Credit Agreement contains financial and operating restrictions that may limit our access to credit. If we fail to comply with its financial or other covenants, we may be required to repay the indebtedness on an accelerated basis, which we may be unable to do and may harm our liquidity and operations;
•failure to establish and maintain effective financial controls could adversely affect our business and stock price;
•we maintain our cash at financial institutions, often in balances that exceed federally insured limits;
•we might require additional capital to fund our current financial obligations and support business growth;
•we are currently subject to securities class action litigation and derivative shareholder lawsuits and may continuebe subject to experiencesimilar or other litigation in the future, which will require significant management time and attention, result in significant legal expenses and may result in unfavorable outcomes, which may have a material adverse impacts as a resulteffect on our business, operating results and financial condition, and negatively affect the price of the COVID-19 pandemic;our common stock;
•we are highly dependent on a limited number of products;
•our long-term growth depends on our ability to develop, acquire and commercialize new products, line extensions or expanded indications;
•we may be unable to successfully commercialize newly developed or acquired products or therapies in the United States;
•demand for our existing portfolio of products and any new products, line extensions or expanded indications depends on the continued and future acceptance of our products by physicians, patients, third-party payers and others in the medical community;
•our commercial success depends on our ability to differentiate the HA viscosupplementation therapies that we own or distribute from alternative therapies for the treatment of OA;
•the proposed down classificationdown-classification of non-invasive bone growth stimulators, including our Exogen, system, by the FDA could increase future competition for bone growth stimulators and otherwise adversely affect the Company’sour sales of Exogen;
•if we are unable to achieve and maintain adequate levels of coverage and/or reimbursement for our products, the procedures using our products, or any future products we may seek to commercialize, the commercial success of these products may be severely hindered;
•our business may be adversely affected if we chooseconsolidation in the healthcare industry leads to acquiredemand for price concessions or invest in new businesses, productsif one or technologies, more Group Purchasing Organizations (“GPO”), third-party payers or other similar entities exclude us from being a supplier;
•we may be unable to complete theseproposed acquisitions or to successfully integrate themproposed or recent acquisitions in a cost-effective and non-disruptive manner;
•if we fail to successfully enter into purchasing contracts for our Surgical Solutions products or engage in contract bidding processes internationally, we may not be able to receive access to certain hospital facilities and our sales may decrease;
•we compete and may compete in the future against other companies, some of which have longer operating histories, more established products or greater resources than we do, which may prevent us from achieving increased market penetration or improved operating results;
•the reclassification of our HA products from medical devices to drugs in the United States by the FDA could negatively impact our ability to market these products and may require that we conduct costly additional clinical studies to support current or future indications for use of those products;
•our ability to maintain our competitive position depends on our ability to attract, retain and motivate our senior management team and highly qualified personnel, and our failure to do so could adversely affect our business, results of operations and financial condition;
•actual or attempted breaches of security, unauthorized access to or disclosure of information, cyberattacks, or other incidents, or the perception that personal and/or other sensitive or confidential information in our possession or control or in the possession or control of our third-party vendors or service providers is not secure, could result in a material loss of business, substantial legal liability or significant harm to our reputation;
•our products and operations are subject to extensive governmental regulation, and our failure to comply with applicable requirements could cause our business to suffer;
•the FDA regulatory process is expensive, time-consuming and uncertain, and the failure to obtain and maintain required regulatory clearances and approvals could prevent us from commercializing our products;
•legislative or regulatory reforms, including those currently under consideration by FDA and the EU, could make it more difficult or costly for us to obtain regulatory clearance, approval or certification of any future products and to manufacture, market and distribute our products after clearance, approval or certification is obtained, which could adversely affect our competitive position and materially affect our business and financial results;
•our HCT/P, which is an acronym for human cell, tissue and cellular and tissue related products, are subject to extensive government regulation and our failure to comply with these requirements could cause our business to suffer;
•if clinical studies of our future products do not produce results necessary to support regulatory clearance, approval or certification in the United States or elsewhere, we will be unable to expand the indications for or commercialize these products;
•interim, “top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data;
•we may be subject to enforcement action if we engage in improper marketing or promotion of our products, and the misuse or off-label use of our products may harm our image in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines and/or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business;
•regulatory reforms, such as the EU Medical Devices Regulation, could limit our ability to market and distribute our products after clearance, approval or certification is obtained and make it more difficult or costly for us to obtain regulatory clearance, approval or certification of any future products, which could adversely affect our competitive position and materially affect our business and financial results;
•recent environmental regulatory actions regarding medical device sterilization facilities could result in disruptions in the supply of certain of our products and could adversely affect our business, results of operations and financial condition;
•if our facilities or those of our suppliers are damaged or become inoperable, we will be unable to continue to research, develop and manufacture our products and, as a result, our business, results of operations and financial condition may be adversely affected until we are able to secure a new facility;
•we depend on certain technologies that are licensed to us. We do not control the intellectual property rights covering these technologies and any loss of our rights to these technologies or the rights licensed to us could prevent us from selling our products, and operations are subject to extensive governmental regulation, and our failure to comply with applicable requirementswhich could causeadversely impact our business, results of operations and financial condition; and
•our principal asset is our interest in BV LLC, and, accordingly, we depend on distributions from BV LLC to suffer;
•wepay our taxes and expenses, including payments under the Tax Receivable Agreement. BV LLC’s ability to make such distributions may be subject to enforcement action if we engage in improper claims submission practicesvarious limitations and resulting audits or denials of our claims by government agencies could reduce our net sales or profits;
•the FDA regulatory process is expensive, time-consuming and uncertain, and the failure to obtain and maintain required regulatory clearances and approvals could prevent us from commercializing our products; our HCT/P products are subject to extensive government regulation and our failure to comply with these requirements could cause our business to suffer;
•if clinical studies of our future products do not produce results necessary to support regulatory clearance or approval in the United States or elsewhere, we will be unable to expand the indications for or commercialize these products; and
•we may be subject to enforcement action if we engage in improper marketing or promotion of our products, that could lead to costly investigations, fines or sanctions by regulatory bodies, any of which could be costly to our business.
restrictions.
PART I
Item 1.Business.
Bioventus Inc. is a Delaware corporation formed on December 22, 2015. Unless the context requires otherwise, in this Annual Report on Form 10-K (Annual Report)(“Annual Report”) the terms “we,” “us,” “our,” the “Company,” “Bioventus,” “Bioventus Inc.” and similar references refer to the combined operations of Bioventus Inc. and its consolidated subsidiaries and affiliates, including Bioventus LLC (BV LLC).
Initial public offering and organizational transactions
On February 16, 2021, we closed an initial public offering (IPO) of 9,200,000 shares of our Class A common stock at a public offering price of $13.00 per share, which included 1,200,000 shares issued pursuant to the underwriters' over-allotment option. We received $111.2 million in proceeds, net of underwriting discounts and commissions, which we used to purchase newly-issued membership interests from (“BV LLC at a price per interest equal to the IPO price of our Class A common stock of $13.00.
Bioventus is a holding company with no direct operations and our principal asset is the equity interest in BV LLC. In connection with the IPO, we completed a series of organizational transactions including, without limitation, the following:
•the limited liability company agreement of BV LLC was amended and restated (Bioventus LLC Agreement) to, among other things, (i) provide for a new single class of common membership interests in BV LLC (LLC Interests), (ii) exchange all of the then existing membership interests of the holders of BV LLC membership interests (Original LLC Owners) for LLC Interests and (iii) appoint Bioventus Inc. as the sole managing member of BV LLC; and
•the acquisition, by merger, of certain members of BV LLC (Former LLC Owners), for which we issued 31,838,589 shares of Class A common stock as merger consideration (Merger)LLC”).
Refer to Note 4 Subsequent events of the Bioventus, Inc. financial statements included in Part II, Item 8. Financial Statements and Supplementary Data of this Annual Report for more information about the above-mentioned transactions as well as the other transactions completed in connection with the IPO (Transactions). Following the completion of the Transactions, Bioventus owned 72.2% of BV LLC. Smith & Nephew, Inc. (Continuing LLC Owner) owned the remaining 27.8% of BV LLC. We have a majority economic interest, the sole voting interest in, and control the management of, BV LLC. As a result, we will consolidate the financial results of BV LLC and will report a non-controlling interest representing the LLC Interests held by the Continuing LLC Owners.
Company overview
We are a global medical device company focused on developing and commercializing clinically differentiated, cost efficient and minimally invasive treatments that engage and enhance the body’s natural healing process. Our devices are most often used to delay or replace the need for an elective surgical procedure and are focused on reaching patients early on in their treatment paradigm. The Company is headquartered in Durham, North Carolina. We have administrative facilities in the United States (U.S.), Canada, and the Netherlands, and a manufacturing facility in the U.S. We directly distribute products in the U.S., Canada, United Kingdom (U.K.), Germany and the Netherlands. In several of these and other markets, we also distribute our products through independent distributors.
We believe our non-invasive medical device and biologic products play a critical role in supporting the body’s own healing mechanisms to heal or eliminate the pain caused by orthopedic conditions and problems, which we define as our active healing products. These products address an estimated $6.0 billion market opportunity across osteoarthritic (OA) joint pain treatment and joint preservation, spinal fusion surgery and bone fractures, each of which is experiencing growth through multiple industry tailwinds, including an aging population, increased participation in sports and active lifestyles and a rise in obesity rates. Our devices are most often used to delay or replace the need for an elective surgical procedure and are focused on reaching patients early on in their treatment paradigm. Our products are widely reimbursed by both public and private health insurers and are sold in the physician’s office or clinic, in ambulatory surgical centers (ASCs), and in the hospital setting in the U.S. and across 37 countries. We manage our business by ourthrough two reporting segments, U.S. and International, which accounted for 91%88% and 9%12%, respectively, of our total net sales during the fiscal year ended December 31, 2020. 2023.
Our portfolio of products is grouped into three areas:
•Pain Treatments is comprised of non-surgical pain injection therapies as well as peripheral nerve stimulation (“PNS”) products to help the patient get back to their normal activities.
•Surgical Solutions is comprised of bone graft substitutes (“BGS”) that increase bone formation to stimulate bone healing in spinal fusions and other orthopedic surgeries, as well as a portfolio of ultrasonic products used for precise bone cutting and sculpting, soft tissue management (i.e., tumor and liver resections) and tissue debridement, in various surgeries, including minimally invasive applications.
•Restorative Therapies is comprised of a bone stimulation system, as well as devices designed to help patients regain leg or hand function due to stroke, multiple sclerosis or other central nervous system disorders.
Financial information regarding our reportable business segments and certain geographic information is included in Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations of this Annual Report. See also Note 15. Segments of the Notes to the Consolidated Financial Statements in and Part II,Item 8. Financial Statements and Supplementary DataData—Notes to the Consolidated Financial Statements—Note 14. Segments of this Annual Report for further information regarding our business segments.
Report. Our existing portfolio of products is grouped into three verticals based on our targeted customer focus:
•OA Joint Pain Treatment and Joint Preservation.We are the largest pure play orthopedics-focused company in the OA joint pain treatment and joint preservation market. We have been the fastest growing hyaluronic acid (HA) participant over the last three years, driving our share to number three by revenue in the U.S. market. We offer the only complete portfolio of HA viscosupplementation therapies, including single, three and five injection regimens, for patients experiencing pain related to OA in the knee. Our HA products are all approved by the U.S. Food and Drug Administration (FDA) through premarket approvals (PMAs), and include:
(a)Durolane, a single injection therapy, was launcheddescribed in the United States in 2018 and is also marketed outside the United States in more than 30 countries including Europe through a CE mark, which is an abbreviation for Conformité Européenne or European Conformity;
(b)GELSYN-3, a three injection therapy, was launched in the United States in 2016; and
(c)SUPARTZ FX, a five injection therapy, was launched in the United States in 2001.
•Bone Graft Substitutes. We are the fastest growing participant in the bone graft substitutes (BGSs) market and offer a broad portfolio of products including human tissue allografts and synthetics. Our BGS products can be used in conjunction with any orthopedic fixation and spinal fusion implant. They are designed to improve bone fusion rates following spinal fusion and other orthopedic surgeries and reduce the need for using the patient’s own bone, which is associated with additional cost and morbidity. Our products include an allograft-derived bone graft with growth factors (OsteoAMP), a demineralized bone matrix (DBM) (Exponent), a cancellous bone in different preparations (PureBone), bioactive synthetics (Signafuse and Interface), a collagen ceramic matrix (OsteoMatrix) and two bone marrow isolation systems (CellXtract and Extractor). Our products have received either 510(k) clearance from the FDA or are marketed pursuant to Section 361 of the Public Health Service Act (PHSA) as Section 361 HCT/Ps. HCT/Ps regulated solelydetail below under Section 361 are human cells, tissues and cellular and tissue-based products that do not require marketing authorization to be marketed in the United States.
•Minimally Invasive Fracture Treatment. Our Exogen system was the number one prescribed device in the bone growth stimulatory market in 2018 (the latest period for which data is available). It has had marketing authorization via a PMA through the FDA for over 25 years. We are the only company to utilize advanced, pulsed ultrasound technology for bone growth in delayed and nonunion fractures in all fracture locations except spine, as well as in fresh fractures of the tibia and radius. Our Exogen system offers significant advantages over electrical based long bone stimulation systems, including a documented mechanism of action, shorter treatment times and superior nonunion heal rates. The system is also sold internationally under a CE mark for nonunions and fresh fractures and is the market-leading bone healing treatment in the delayed union and nonunion market in Japan.
The COVID-19 pandemic and the measures imposed to contain the spread of the virus disrupted our business beginning in early March 2020 as healthcare systems across the U.S. were forced to limit patient visits and elective surgical procedures. The effects of the pandemic began to decrease in late April 2020 and we saw a very strong recovery for our products at the end of the second quarter as restrictions on orthopedic procedures were lifted across the United States and patients also returned to orthopedic offices. Refer to Part I, Item 1A. Risk Factors and Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations for more information regarding the impact and related risks of the COVID-19 pandemic on our business.“Our products.”
Our growth strategy
We intend to pursue the following strategies to build a market-leading and customer-focused company centered on the OA joint pain treatmentour three product groupings, Pain Treatments, Surgical Solutions and joint preservation, BGSs and minimally invasive fracture treatments, andRestorative Therapies, to continue to grow our net sales and Adjusted EBITDA:deliver profitable growth:
•Continue to expand market share in HAHyaluronic Acid (“HA”) viscosupplementation.We intend to increase sales of our HA viscosupplementation therapies and extend our market leadership in this category by building on our unique positioning as the only company to offer a one, three and five injection treatment regimen and by expanding payer coverage, which weregimen. Our product, Durolane, is known to have done successfully, increasing the number of lives under contract from 6 million to 48 million between 2017 and 2020. This increase in lives, along with our differentiated portfolio and dedicated direct sales team, has allowed us to achieve significant market share gains over the last several years and positioned us as the largest pure play orthopedic-focused companyhighest molecular weight in the U.S. HA viscosupplementationsingle injection market, with a market share of approximately 17% as of September 26, 2020.which is an important differentiator.
•Introduce new OA joint pain treatment and joint preservation products. To expand our offering beyond HA viscosupplementation therapies and build a comprehensive portfolio for the OA joint pain treatment and joint preservation, we are planning to commercially launch a range of new therapies over the next several years, including:
(a)Agili-C. An off-the-shelf aragonite implant designed for implantation into osteochondral defects in the knee. The Agili-C implant received breakthrough device designation by the FDA in the fourth quarter of 2020. We have an option to acquire this technology from CartiHeal upon FDA approval. CartiHeal submitted the non-clinical module of the PMA in January 2021 and expects to submit the final, clinical module of a Modular PMA in the fourth quarter of 2021 seeking FDA approval.
(b)MOTYS. A placental tissue injectable biologic for knee OA, which we began selling in the cash pay market in the fourth quarter of 2020 as a Section 361 HCT/P pursuant to a temporary FDA policy of enforcement discretion. On October 29, 2020, we received FDA confirmation indicating its authorization of our investigational new drug application (IND), and a clinical trial for MOTYS has commenced in the first quarter of 2021. In parallel, we plan to pursue a required BLA premarket approval for this product, which we expect would expand insurance payment alternatives over time.
(c)PROcuff. A bio-inductive collagen implant for regeneration of tendon tissue in the rotator cuff. We expect to file a request for 510(k) clearance in either the second or third quarter of 2022.
•Further develop and commercialize our BGSsurgical solutions portfolio. We intend to grow our presence in the BGS marketSurgical Solutions target markets and expand our reach into the operating room in both ASCs and hospitals.as we look to commercialize recent product launches of Bone Scalpel Access for minimally invasive spine procedures. In the near-term, we plan to maintain and selectively expand our profitable product lines, by adding todriving profitability through the expansion of our U.S. distributor base in an effort to reach significantly underpenetrated markets. Over time, weand direct surgical sales team including the introduction of a Capital Sales Team. We intend to launch product line enhancements and invest in the development of next-generation BGSsurgical solution therapies to continue to grow our market share. Consistent with this strategy, we recently launched the Signafuse Bioactive Strip and anticipate launching the OsteoAmp Flowable in 2021.
•Expand indications for use for our Exogen system.We are focused on generating incremental clinical data and peer-reviewed publications to expand our indications and continue to grow our market leading share. We are currently underway with the B.O.N.E.S. clinical studies, which are aimed at generating data to support label expansion in additional bone types and expanded reimbursement for the treatment of fresh fractures in patients at risk of nonunion due to certain comorbidities, such as diabetes or obesity. We commenced patient enrollment to study three specific bones in 2017 and began a rolling release of data in late 2020. Depending on the results from our studies, we plan to submit a total of three PMA supplements to the FDA, the first of which was submitted in December 2020 seeking approval for the adjunctive treatment of acute and delayed metatarsal fractures to reduce the risk of non-union. We plan to submit the second PMA supplement in the second quarter of 2022 and the third PMA supplement in the second half of 2023.
•Invest in research and development. We are focused on internal researchbroadening and development to broadeninnovating our portfolio of therapies to manage OA joint painproducts, and joint preservation, expand our Exogen system product label and undertake clinical research to support commercialization of our next-generation of BGS products. We see significant opportunity to develop innovative and clinically differentiated products internally with our qualified research and development team. We rely on a small team of 40 highly trained individuals to develop new products, conduct clinical investigations and help educate health carehealth-care providers on using our products. Our researchproducts and development team is comprised of 13 members holding PhDs and 18 members with more than 20 years of experience inprioritize the medical device industry. We collaborate with academic centers of excellence, leading contract research organizations and other industrial groups to complement and expedite execution of our research and development programs and minimize fixed costs.
•Pursue business development opportunities. Consistent with our track record of partnerships and acquisitions of MOTYS, PROcuff and CartiHeal, we intend to continue to pursue business development opportunities that leveragecould drive the biggest benefit for patients, customers, and our significant customer presence across orthopedics, broaden our portfolio and increase our global footprint. We will continue to search for clinically differentiated and cost-effective products and technologies that also balance our portfolio in terms of risk and time to market.business.
•OpportunisticallyStrategically grow our international markets.We intend to focus our international business on current markets which present the greatest growth opportunities, and where our existing portfolio can maintain and increase profitable growth over time, either through direct or distributor based channels. For example, we launched OsteoAMP in Canada in 2020, where Durolane and Exogen had a market leading presence in 2016. We also plan to selectivelystrategically expand to new markets with Durolane, Exogen and our BGSsexisting portfolio and intend to pursue further new market opportunities in the Asia Pacific markets. In particular, China represents an attractive and exciting market given its large and aging population as well as its rising middle class. We have added management in China and will be creating a legal entity as we seek approval from the China Food and Drug Administration for Durolane, which we believe will be facilitated by the successful completion of our Chinese randomized controlled trial (RCT).
Recent Developments
Investment and Potential Acquisition
On January 4, 2021, we made a convertible debt investment of $1.5 million, (Investment), in a medical device company (Target), as a part of our exclusive negotiations with the Target regarding the Potential Transaction (as defined below). If the Potential Transaction is not consummated, the Investment will be convertible into equity of the Target at our discretion or upon a change of control of the Target, which we expect to result in approximately 2% equity ownership in the Target. The arrangement has allowed the parties to explore a possible acquisition of the Target (Potential Transaction) for a payment of $45.0 million to be paid at closing with contingent payments of up to $65.0 million to be paid upon achievement of certain key milestones. While still subject to due diligence, we believe the Target’s patent-protected portfolio of FDA approved products and its product development pipeline are highly complementary to our OA joint pain treatment and joint preservation vertical and would allow us to further leverage our claims processing infrastructure. For the year ended December 31, 2020, the Target generated approximately $40.0 million in revenue and had loss from operations of approximately $14.0 million. We expect the Target to be accretive to revenues immediately if the Potential Transaction closed, and to have a positive contribution to combined Company net income excluding purchase accounting and transaction costs by the end of year one post-close. We were notified that a minority shareholder of the Target filed a complaint with the Court of Chancery of the State of Delaware on February 4, 2021 contesting the Potential Transaction, as well as related motions to expedite proceedings and issue a temporary restraining order. We understand that the Target was successful defending the action and on March 4, 2021, the complaint and motions were dismissed, but there can be no assurance that this action will not impact the timing, terms, or likelihood of closing of the Potential Transaction. Any resulting acquisition will be funded through our current cash balance and, if necessary, proceeds from our existing line of credit. We cannot assure that the acquisition will occur on or before a certain time, on the terms described herein, or at all. See Part I, Item 1A. Risk Factors—Risks related to our business—If we choose to acquire or invest in new businesses, products or technologies, we may be unable to complete these acquisitions or to successfully integrate them in a cost-effective and non-disruptive manner.selectively.
Our products
We offer a diverse portfolio of active healing products to serve physicians spanning the orthopedic continuum, including sports medicine, total joint reconstruction,knee, hand and upper extremities, foot and ankle, podiatricpodiatry, trauma, general surgery, trauma, spine and neurosurgery, in the physician’s office or clinic, ASCsambulatory surgical centers (“ASCs”) or in the hospital setting.
Our portfolio of products is grouped into three verticalsareas based on clinical use: (i) OA joint pain treatment and joint preservation,Pain Treatments, (ii) BGSsSurgical Solutions and (iii) minimally invasive fracture treatment.
OA joint pain treatment and joint preservation
Knee OA is a degenerative condition that is chronic in nature and is characterized by gradual breakdown and destruction of the cartilage in the knee. This condition develops over years and is often found in patients who exhibit joint malalignment, have had a joint injury, or are overweight. The disease can involve joint inflammation and results in symptoms that include redness, warmth, swelling, stiffness, tenderness, limited range of motion and pain. As the condition advances, the knee joint gradually loses cartilage tissue and the cartilage layer attached to the bone deteriorates to the point where eventually the bone becomes exposed.
We have the only complete one, three and five injection portfolio in the HA viscosupplementation market in the United States with Durolane, GELSYN-3 and SUPARTZ FX.
| | | | | | | | | | | | | | | | | | | | |
Product | | Description | | Regulatory pathway | | Region where marketed(1)
|
| | Single injection HA
viscosupplementation therapy | | •PMA
•Device approval by Health Canada
•CE mark and other registrations(2)
| | •United States
•Canada
•Europe
|
| | | | | | |
| | Three injection HA
viscosupplementation therapy | | •PMA
| | •United States
|
| | | | | | |
| | Five injection HA
viscosupplementation therapy | | •PMA
| | •United States
|
__________________Restorative Therapies.
(1)We maintain exclusive distribution agreementsPain Treatments
Our Pain Treatment products include hyaluronic acid-based (“HA”) products for knee osteoarthritis and peripheral nerve stimulation (“PNS”) devices. Our HA products are designed to work with respectthe body’s biological processes, providing a natural lubricant into the joint and providing relief for mild to Durolane, GELSYN-3moderate pain, improving mobility, and SUPARTZ FXhelping the patient return to their normal activities. Our PNS product targets peripheral nerve pain at its source without the use of drugs and its small profile allows the system to be implanted in many locations on the United States. We maintain exclusive distribution agreements and own certain assets with respect to Durolane outside the United States.body, depending on patient needs.
(2)
Durolane is also approved for marketing in Argentina, Australia, Brazil, Columbia, India, Indonesia, Jordan, Malaysia, Mexico, New Zealand, Russia, Switzerland, Taiwan, Turkey and the United Arab Emirates (UAE).
Single Injection Therapy
Durolaneis aan FDA-approved, sterile, transparent and viscoelastic gel that is a single injection therapy that is indicated in the United States for the symptomatic treatment of OAosteoarthritis (“OA”) in the knee in the United States.patients who have failed to respond adequately to conservative non-pharmacological therapy and simple analgesics. Durolane is also indicated in certain markets outside the United States for the hip, ankle and shoulder, as well as for treatment of other small orthopedic joints outside the United States.joints. Durolane contains high levels of HA and is injected directly into the joints affected by OA to relieve pain and restore lubrication and cushioning. This may improve joint function and help to potentially avoid or delay knee replacement surgery.
Physicians administer Durolane to the affected knee joint in a single injection and it has been observed to provide a benefit for pain reduction in patients with OA in the knee for up to 26 weeks. Durolane’s injection schedule results in economic advantages and greater patient convenience and compliance compared to other HA viscosupplementation therapies which require weekly injections over a period of three to five weeks. For example, we believe that changes in physician visiting patterns, as a result of the COVID-19 pandemic, have led to increased preference for single injection therapies.
Durolane is highly purified and based upon a natural and patented non-animal stabilized HA (NASHA)(“NASHA”), expanding use to patients who are allergic to animal derivedanimal-derived solutions.
Comparison of major FDA-approved single injection HA viscosupplementation therapies
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Product
Manufacturer or
distributor
| | Indication | | Source and process | | Active ingredient /
treatment dosage | | Duration |
Bioventus
| | OA of the knee | | Non-animalstabilized HA | | NASHA / (60 mg) | | Six months |
| | | | | | | | |
Synvisc-One
Sanofi S.A.
| | OA of the knee | | Animal sourced Hylan A and Hylan B polymers | | Hylan G-F 20 / (48 mg) | | Six months |
| | | | | | | | |
Monovisc
DePuy Orthopaedics,
Inc.
| | OA of the knee | | Non-animal cross-linked sourced HA | | 2.2% sodium hyaluronate / (88 mg) | | Six months |
| | | | | | | | |
Gel-One
Zimmer Biomet
Holdings, Inc.
| | OA of the knee | | Animal sourced HA | | 1.0% sodium hyaluronate /(30 mg) | | Three months |
Durolane clinical data
Durolane’s proprietary stabilizing technology substantially extends the amount of time it remains in the joint. Multiple studies have been conducted to determine Durolane’s half-life, which is the amount of time needed for 50% of the injected material to be broken down and excreted from the body.
In one study, Durolane’s half-life in the joint was studied in a rabbit model. Results showed the Durolane remained in the joint with an observable half-life of 32 days, substantially longer than the half-lives of Synvisc and unmodified HA, as determined in comparable studies, which were 1.5 days and less than 1 day, respectively.
The long half-life of Durolane was also observed in the 2002 Lindqvist et al. human study where six healthy volunteers were given a single injection of Durolane that contained a radioactive isotope that could be traced, allowing scientists to measure Durolane’s elimination from the body over time. The results showed a 30-day half-life, indicative of the expected long residence time in the joint due to Durolane’s proprietary stabilizing technology and preclinical studies.
In terms of efficacy, Durolane has been directly compared against the main intra-articular therapeutic options available for managing osteoarthritic pain: SUPARTZ FX, a five injection product, Synvisc One, a single injection product and methylprednisolone acetate, an intra-articular corticosteroid.
In a multi-center randomized, blinded, controlled trial of 349 patients with mild-to-moderate knee OA, Durolane was compared with SUPARTZ FX. This 2015 Zhang et al. study concluded that one injection of Durolane was non-inferior to five weekly injections of SUPARTZ FX in terms of pain, stiffness, physical function and global self-assessment.
In an independent, investigator-initiated randomized, controlled study involving 213 patients with mild-to-moderate knee OA, Durolane was further compared to Synvisc-One. After following up with the patients over a span of 12 months following the treatment, the results from this 2013 McGrath et al. study showed that Durolane produced significantly more durable pain relief effects than Synvisc-One, while also providing longer-lasting improvements in range of motion and a reduction in the use of pain medication for study participants.
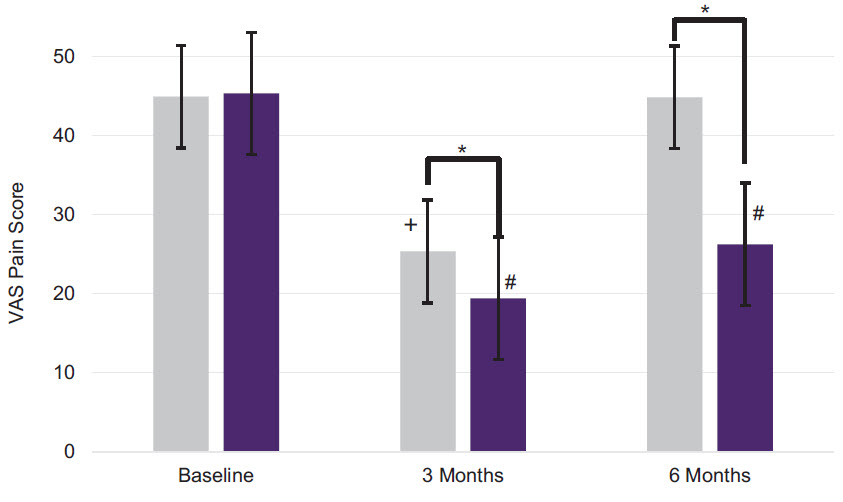
Greater Reduction in Knee Pain versus Synvisc-One
| | | | | |
| *p<0.000 compared to Synvisc
|
| #p<0.000 compared to DUROLANE baseline
|
| *p=0.008 compared to Synvisc baseline
|
(n=168)
Mean values +/- standard deviation
In a separate prospective, multi-center, randomized, active-controlled, double-blind, non-inferiority clinical trial with 442 enrolled patients with knee OA, it was observed that single injection Durolane was well tolerated and non-inferior compared to the corticosteroid methylprednisolone acetate at twelve weeks. Methylprednisolone acetate is a steroid injectable formulation used to treat pain and swelling that occurs with OA and other joint disorders. The effect size for pain, physical function and stiffness scores favored Durolane over methylprednisolone acetate from twelve to 26 weeks. The benefit of Durolane was maintained through 26 weeks, while that of methylprednisolone acetate declined during the same period. An additional injection of Durolane at 26 weeks conferred improvements through 52 weeks without increased sensitivity or risk of complications compared to the initial injection. One subset of 31 patients treated with Durolane remained pain free after six months from the first injection and did not elect to receive a second injection.
As of December 31, 2020, over 2 million injections of the Durolane formulation have been safely administered globally since its international launch in 2006. We launchedcurrently market Durolane in the United States in March 2018 and have owned certain Durolane assets outside of the United States relating to trademark, product registrations and clinical data since November 2015.Europe.
Three Injection Therapy
GELSYN-3GELSYN-3™ is an FDA-approved sterile, buffered solution of highly purified sodium hyaluronate that is administered as a three injection HA viscosupplementation therapy. It is indicated for the treatment of pain due to knee OA in patients who have failed to respond adequately to conservative non-pharmacologic therapy and simple analgesics. The solution treats knee OA by providing temporary replacement for the diseased synovial fluid and restoring the lubricity of bearing joint surfaces. Physicians administer GELSYN-3 to the affected knee joint once a week for three consecutive weeks. GELSYN-3 provides relief of knee pain and may help delay the need for total knee replacement surgery. GELSYN-3 is derived from bacterial fermentation, is highly purified and does not involve the use of animal products, thereby reducing the potential risk of an immune response following injection. We currently market GELSYN-3 in the United States. As of December 31, 2020, approximately 900,000 injections of the GELSYN-3 HA formulation have been safely administered in the United States since its launch in 2016.
GELSYN-3 clinical data
The safety and efficacy of GELSYN-3 was assessed in a prospective, multicenter, randomized, controlled, double-blind, non-inferiority pivotal study that enrolled 381 adult patients with knee OA. Patients were randomized to receive three weekly injections of GELSYN-3 or three weekly injections of Synvisc 3, a three injection regimen commercialized in the United States by Sanofi S.A., with follow-up visits scheduled up to 26 weeks. GELSYN-3 was observed to be non-inferior to Synvisc 3 at the 26-week time point.
Five Injection Therapy
SUPARTZ FXSUPARTZ™ is an FDA-approved sterile and viscoelastic solution of HA that is administered as a five injection HA viscosupplementation therapy. It is indicated for the treatment of pain in patients with knee OA who failed to adequately respond to conservative nonpharmacological therapy and simple analgesics. The solution treats knee OA by providing temporary replacement for the diseased synovial fluid and restoring the lubricity of the bearing joint surfaces. Physicians administer SUPARTZ FX to the affected knee joint once a week for five consecutive weeks. SUPARTZ FX may also delay the need for total knee replacement. SUPARTZ FX is derived from HA extracted from certified and veterinary inspected chicken combs. Risks can include general knee pain, warmth and redness or pain at the injection site. We currently market SUPARTZ FX in the United States. As of December 31, 2020, over 410 million injections of the SUPARTZ FX HA formulation have been safely administered globally since its launch in 1987.
SUPARTZ FX clinical data
In a double blind, randomized, multi center, parallel group study conducted by Day et al. in 2004 of the effectiveness and tolerance of intra-articular SUPARTZ FX compared to control (saline) treatment for knee OA, it was observed that SUPARTZ FX reduced knee pain in patients during the post-injection period by about 50% from the baseline. Of 240 patients randomized for inclusion in the study, 223 patients were evaluable for the modified intention to treat analysis and the statistically significant difference from the control was apparent after the series of injections was complete. Intra-articular SUPARTZ FX therapy was shown to be more effective than saline in mild to moderate knee OA for the 13-week post injection period of the study.
The safety and efficacy of SUPARTZ FX was observed by Strand et al. in an integrated analysis. This integrated analysis included five separate double-blind, randomized, saline-controlled trials, and included a total of 1,155 patients comparing five weekly injections of SUPARTZ FX versus a saline placebo. The pooled results from this study showed that SUPARTZ FX produced statistically significantly greater reduction from baseline in total Lequesne scores, a measure of overall function including pain. The incidence of adverse events were observed to be minimal and similar in both treatment arms. Furthermore, none of the reported adverse events were observed to be deemed treatment-related suggesting that SUPARTZ FX was safe and well-tolerated.
Comparison
Our StimRouter® Peripheral Nerve Stimulation (“PNS”) system is a permanent option that provides relief for chronic peripheral pain, including nerve pain, neuroma, neuropathic pain, post-stroke shoulder pain and neuralgia. StimRouter is implanted during a minimally invasive outpatient procedure performed under local anesthetic and delivers gentle electrical pulses directly to target peripheral nerve pain at its source. Its small profile allows the system to be implanted in many locations around the body, depending on patient needs. StimRouter is ideally suited for patients with chronic pain of FDA-approved multi-injection HA viscosupplementation therapiesa peripheral origin who are unable to find sustained pain relief with other treatment options such as nerve blocks, nerve ablation, and other temporary treatments. StimRouter is programmed with up to eight different stimulation programs from which the patient is able to select, turn off/on and increase or decrease the stimulation intensity.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Product
Manufacturer or distributor
| | Indication | | Source and
process | | Active ingredient /
total treatment
dosage | | Number of
injections
per course | | Duration |
| | | | | | | | | | |
Bioventus
| | OA of the knee | | Fermented, bacterial derived HA | | 0.84% sodium hyaluronate
(50.4 mg) | | Three | | Six months |
| | | | | | | | | | |
Bioventus
| | OA of the knee | | Naturally derived, purified HA | | 1.0% sodium hyaluronate
(75/125 mg) | | Three to Five | | Six months |
| | | | | | | | | | |
Synvisc
Sanofi S.A.
| | OA of the knee | | Hylan polymers, purified HA | | 0.8% Hylan G-F
20 (48 mg) | | Three | | Six months |
| | | | | | | | | | |
Euflexxa
Ferring
Pharmaceuticals Inc.
| | OA of the knee | | Fermented, bacterial derived HA | | 1.0% sodium hyaluronate
(60 mg) | | Three | | Six months |
| | | | | | | | | | |
Hyalgan
Fidia Farmaceutici
S.p.A.
| | OA of the knee | | Naturally derived, purified HA | | 1.0% sodium hyaluronate
(60 mg/100 mg) | | Three to Five | | Six months |
| | | | | | | | | | |
Genvisc-850
OrthogenRx, Inc.
| | OA of the knee | | Fermented, bacterial derived HA | | 1.0% sodium hyaluronate
(75/125 mg) | | Three to Five | | Six months |
Developmental and clinical pipeline for Pain TreatmentsDevelopment and Clinical Pipeline
Amniotic tissue products for the treatment of OA
Collaboration and development agreement for MOTYS
On May 29, 2019, we entered into a Development Agreement with Musculoskeletal Transplant Foundation, Inc. (MTF) to develop an injectable placental tissue product, MOTYS, for use in the OA joint pain treatment and joint preservation.
The developmentTalisMann® Pulse Generator and commercialization of the product will take place in two stages, and we began limited commercialization of MOTYS to a cash pay only market in the fourth quarter of 2020 as a Section 361 HCT/P pursuantReceiver (not yet cleared by FDA) is an accessory to the FDA’s policy of enforcement discretion allowing for marketing without the required BLA approval until May 2021, while in parallel we pursue a BLA pre-market approval for the product. Once approved as a biologic, MOTYS will be eligible for health insurance reimbursementStimRouter® PNS system and hence gain access a broader patient population. We are planningis designed to conduct randomized clinical trials to ultimately support the submissionprovide more powerful stimulation to the FDAtargeted peripheral nerve, potentially enabling physicians to address chronic pain of a BLA for the use of MOTYSperipheral nerve origin in the OA joint pain treatment.
Based on our preclinical evidence, we believe the MOTYS formulation holds potential for mitigating OA joint pain while protectinglarger, deeper, or damaged cartilagenerves. TalisMann has a small profile and promoting anti-catabolic and pro-anabolic events that could ultimately result in delayed disease progression in OA. We have completed extensive in vitro and in vivo studies comparing the effect of MOTYSis attached to the clinical standard of care (steroid injections). MOTYS provided non-inferior pain relief effects to a steroid, but was superior in its effect on cartilage protectionStimRouter lead intraoperatively and in promotion of new tissue formation.pocketed under the skin after the StimRouter lead electrodes are placed near the targeted peripheral nerve.
In October 2020, we received FDA confirmation indicating its authorization of our IND and plan to initiate clinical studies by year end. Amniotic products have been extensively and safely used in clinical practice, and FDA has granted Regenerative Medicine Advanced Therapy (RMAT), designation to other amniotic tissue products being investigated for use in OA, which enables an expedited development pathway as well as eligibility for increased and earlier interactions with FDA. We intend to submit a request for RMAT designation for MOTYS in 2022.
Implantable for the treatment of rotator cuff injuries
Development collaboration agreement for PROcuffTrice Medical, Inc.
On August 23, 2019, we entered into an exclusive Collaboration Agreement with Harbor Medtech Inc. (Harbor) to develop and license the rights to commercialize a woven-suture-collagen composite implant product, PROcuff, for the regeneration of tendon tissue. Concurrently with the execution of the agreement, we purchased $1.0 million of shares of Harbor. As a result of Harbor’s achievement of certain milestones, on October 5, 2020, we purchased $1.0 million of additional shares of Harbor. The sole use of proceeds from these investments is for the development of the woven-suture-collagen composite implant product and we have the right to purchase the product from Harbor once it is cleared for marketing by the FDA.
We have currently completed a pilot sheep implantation study through a collaboration with a prominent academic investigator. Results indicate that the material is well tolerated, rapidly integrated and promotes the formation of new tendon tissue at the bone tendon interface. We expect to file a request for 510(k) clearance in either the second or third quarter of 2022. We plan to conduct post-clearance human clinical studies with the composite implant to further demonstrate the safety and efficacy of the product, and facilitate reimbursement.
Treatment of Cartilage for Osteochondral defects
CartiHeal (developer of Agili-C) investment and option and equity purchase agreement
On July 15, 2020,2021, we made a $15.0 million equitystrategic investment in CartiHeal (2009) Ltd. (CartiHeal),Trice Medical, Inc. (“Trice”). Trice is a privately-heldprivately held company headquarteredthat develops and commercializes minimally invasive technologies for sports medicine and orthopedic surgical procedures. Trice combines its handheld arthroscope and portable ultrasound visualization technologies with its surgical devices to treat a range of sports medicine and orthopedic conditions, including tendinopathy, planter fasciitis and carpel tunnel, in Israelorder to improve patient recovery time, reduce pain, minimize scarring and developermove surgical procedures out of higher cost points of care. Trice’s established and growing presence in sports medicine and orthopedics is directly aligned with our strategy of expanding our offerings. Our investment resulted in exclusive sales and distribution rights to Trice’s products outside of the proprietary Agili-C implant for the treatmentUnited States.
Surgical Solutions
Our Surgical Solutions product portfolio is comprised of joint surface lesions in traumaticclinically efficacious and osteoarthritic joints.
We believe Agili-C is the only product in clinical development in the United States as an off-the-shelf scaffold implant that is designedcost-effective bone graft solutions to regenerate hyaline cartilage and subchondral bone simultaneously. The associated surgical procedure is similar to osteochondral allograft implantation, but ismeet a single-step process and is easier, faster and more cost-effective. We believe this is the first cartilage repair technology to be tested in trials designed for regulatory approval in the United States in non-OA and OA patients, potentially unlocking applications for millionsbroad range of patients with knee OA and cartilage defects. We also believe Agili-C will enable the treatment of cartilage lesions in a significant population of OA patients, including those younger, active patients for whom available treatment options are limited. The FDA’s grant of breakthrough device designation in the fourth quarter of 2020 for the treatment of an International Cartilage Repair Society (ICRS) grade III or above knee-joint surface lesions(s), with a total treatable area of 1-7cm2, without severe osteoarthritis (Kellgren-Lawrence grade 0-3) is a promising development, as such designation may help patients receive more timely access to Agili-C by expediting its development, assessment and review by the FDA. On January 12, 2021, the Centers for Medicare and Medicaid Services (CMS) issued a final rule under which a breakthrough device designation by the FDA also provides a streamlined pathway to national Medicare coverage for a period of four years, beginning as early as the FDA approval for the product. On March 12, 2021, CMS delayed by 60 days the effective date of the final rule on Medicare coverage for innovative technology, which was previously slated to become effective March 15, 2021. The agency also provided a 30-day public comment period, which ends April 16, 2021. We believe Agili-C also has the potential for broader indications for use in other joints, providing entrance into the global market for cartilage repair products designed to delay or eliminate the need for knee replacements.
In preclinical studies Agili-C was associated with osteochondral regeneration, good lateral integration and hyaline cartilage formation in critical size defects at 20 months when implanted in a goat, with the implant being fully resorbed between six to 20 months. The Agili-C implant has been implanted in more than 190 patients outside the United States with follow up of more than four years and is CE marked. The implant is currently being evaluated in a pivotal study pursuant to an IDE filed with the FDA. The trial’s objective is to demonstrate the superiority of the Agili-C implant over the surgical standard of care (microfracture and debridement) for the treatment of cartilage or osteochondral defects, in both osteoarthritic knees and knees without degenerative changes. The study’s protocol design, which is based on feedback from multiple pre-IDE interactions with the FDA, involves broad inclusionary criteria, such as defect size, age, and etiology, multiple controls, including microfracture and debridement, and multiple pre-planned secondary endpoints. The study has an adaptive design, which allows for a maximum of 500 planned patients, includes multiple interim analyses to estimate sample sizepatient needs and includes European Union (EU), Israeli and U.S. sites.
Our CartiHeal investment follows the recently completed enrollment and reporting of interim results in CartiHeal’s IDE multinational pivotal study for Agili-C. This investmentis expected to enable CartiHeal to complete the study, including all patient follow-up, and submit a PMA to the FDA. Under the equity purchase agreement, CartiHeal can secure an additional $5.0 million from us, if needed, for IDE study completion. We previously made an initial $2.5 million investment in CartiHeal in January 2018 and a subsequent investment of $0.2 million in January 2020 as part of prior CartiHeal financing rounds. Any additional investment we make will be subject to customary closing conditions.
Concurrent with the July 15, 2020 investment, we entered into an Option and Equity Purchase Agreement with CartiHeal and its shareholders, which provides us with an exclusive option to acquire 100% of CartiHeal’s shares, or the Call Option, and provides CartiHeal with a put option that would require us to purchase 100% of CartiHeal’s shares under certain conditions, or the Put Option. The Call Option is exercisable by us at any time after the closing of the investment. The Put Option is only exercisable by CartiHeal upon pivotal clinical trial success, including achievement of certain secondary endpoints and FDA approval of the Agili-C device with a label consistent in all respects with pivotal clinical trial success.
If not previously exercised, the Call Option and the Put Option terminate 45 days following the FDA approval of Agili-C or in the event of failure of the pivotal clinical trial. We also have the right to terminate the Call Option and Put Option at any time ending 30 days after receipt from CartiHeal of the statistical report regarding the final results of the pivotal clinical trial upon payment of a breakup fee of $30.0 million. Consideration for the acquisition of all of the shares of CartiHeal pursuant to the Call Option or Put Option would be $350.0 million, all of which would be payable at closing, with an additional $150.0 million payable upon achievement of certain sales milestones related to Agili-C.
Bone Graft Substitutes
BGSs in spinal fusion and other procedures
procedures. Bone grafting is a surgical procedure used to fusepromote fusion of spinal vertebrae, replace missing bones,fill bone voids, fix bones that are damaged from trauma or problem joints, or to facilitate growing bones around an implanted device, such as aspinal hardware (i.e., cages and rods), total knee replacement. The bones used in areplacements and long bone graft can come from a particular patient’s own body, referred to as an autograft, or from a donor, referred to as an allograft, or can be entirely man-made, referred to as a synthetic. Most bone grafts are expected to be reabsorbed and replaced as the natural bone heals over a few months.
Our BGS product portfolio is comprised of clinically efficacious and cost effective bone graft solutions to meet a broad range of patient needs and procedures.fixation. Our products are designed to improve bone fusion rates following spinalspine and other orthopedic surgeries, including trauma and reconstructive foot and ankle procedures. Our portfolio is also comprised of an ultrasonic surgical system. These products includeare used for precise bone cutting and sculpting, soft tissue management (i.e., tumor and liver resections) and tissue debridement, in various surgeries including minimally invasive applications, primarily in the areas of neurosurgery, orthopedic surgery, general surgery, wound, plastics/reconstruction, and cranio-maxillo-facial surgery.

OSTEOAMP® is an allograft-derived bone graft with growth factors (OsteoAMP)used for orthopedic, neurosurgical and reconstructive bone grafting procedures. OSTEOAMP is an allogeneic bone graft that is available in multiple formats (fibers, putty, sponge and granules) that is processed with bone marrow cells to maintain the wide array of growth factors present in native bone. We currently market OSTEOAMP in the United States. We launched OSTEOAMP Flowable in 2021, which is designed to be moldable and easy to use, with a convenient, ready to use syringe. Additionally, a customized cannula-based delivery system is currently in development, which is designed to enhance delivery of the product further enabling use in minimally invasive surgical procedures. FDA 510(k) submission for the cannula-based delivery system was accepted during the fourth quarter of 2023. We have successfully implanted almost half of the subjects for our Level-I study of OSTEOAMP vs. Infuse and continue to drive enrollment of the study.
EXPONENT provides an osteoconductive scaffold with osteoinductive potential while providing optimal handling characteristics indicated for posterolateral spine procedures. EXPONENT is derived from human allograft bone tissue and is combined with a migration-resistant resorbable carrier and formulated into a putty that is ready-to-use out of the syringe. EXPONENT is highly malleable and easy to mold and pack into the surgical defect. Donor bone is sourced from AATB-certified and FDA-registered tissue banks in the United States. All tissues are screened for the standard panel of infectious viruses. We currently market EXPONENT in the United States.

PUREBONE provides a natural osteoconductive scaffold that facilitates cellular ingrowth and revascularization which is indicated for orthopedic, neurosurgical and reconstructive bone grafting procedures. PUREBONE is 100% human bone, and is available as demineralized cortical fibers, demineralized cancellous strips and blocks, and mineralized cancellous chips. Demineralized cortical fibers are easy to mold, shape and pack, and provide osteoinductive potential. The fibers demonstrate high fluid retention and expansion properties, which potentially increases the opportunity for bone-on-bone contact. Demineralized block and strip formats provide interconnected porosity with compressible, sponge-like handling characteristics, and provide osteoinductive potential. Mineralized cancellous chips range from 1-4 mm and 4-10 mm granule size for optimal void packing capabilities. Demineralized PUREBONE formats provide osteoinductive potential to recruit and differentiate bone-forming cells. Donor bone is sourced from AATB-certified and FDA-registered tissue banks in the United States. All tissues are screened for the standard panel of infectious viruses. We currently market PUREBONE in the United States.

SIGNAFUSE contains a synergistic combination of biomaterials that supports new bone formation which is indicated for standalone posterolateral spine, extremities and pelvis, as well as a bone graft extender in the posterolateral spine. SIGNAFUSE is a synthetic bone graft made up of bioglass and a biphasic mineral (60% hydroxyapatite, 40% β-tricalcium phosphate) available in putty and strip formats. Bioactive synthetic bone graft substitute is comprised of a mixture of calcium phosphate granules and bioglass granules suspended in a resorbable polymer carrier that facilitates handling and delivery of the granule components to fill spaces of missing bone. The unique and synergistic combination of biomaterials in SIGNAFUSE is designed to help accelerate cellular activity and kick-start osteogenesis. Bioventus has recently received FDA clearance for expanded indications for the use of SIGNAFUSE in spinal procedures, specifically for filling cages. We expect this expanded indication will continue to drive sales of the product. We currently market SIGNAFUSE in the United States.

INTERFACE is designed to facilitate a rapid biologic response that stimulates the bone healing process and is used for posterolateral spine when mixed with autograft, extremities and pelvis. INTERFACE’s patented particle technology is designed for enhanced bone graft performance through irregularly shaped synthetic bioglass granules that provide an osteoconductive scaffold for new osseous ingrowth and tissue generation. The patented bioglass component stimulates the formation of an apatite layer as early as seven days after application on the surface of the granules. The apatite surface layer that is formed is equivalent in composition and structure to the hydroxyapatite found in bone and provides an osteoconductive bioactive scaffold that supports the generation of new osseous tissue. New bone infiltrates around the granules, allowing the repair of the defect as the granules are absorbed. The patented INTERFACE Bioactive Bone Graft particle size of 210-420 microns is designed for a faster speed of bone fill than glass particles with a broader particle size distribution of 90-710 microns and smaller particles below 210 microns. INTERFACE features consistent composition without variability inherently found in particle size and porosity of tissue based grafts. INTERFACE Bioactive Bone Graft conforms to ASTM specification F1538 for 45S5 bioactive glass. INTERFACE is packed in a sterile, single use vial. We currently market INTERFACE in the United States.

OSTEOMATRIX+ is a synthetic bone graft with exceptional handling, rapid hydration and a biphasic composition for sustained performance used on the posterolateral spine, extremities and pelvis. OSTEOMATRIX+ is a moldable bone graft substitute consisting of biphasic granules designed to produce a reliable, porous scaffold and sustained osteoconductivity throughout bone remodeling. The OSTEOMATRIX+ biphasic granules are composed of 60% hydroxyapatite and 40% beta-tricalcium phosphate (“β-TCP”), a DBM (Exponent), cancellousratio demonstrated to have advantageous bone remodeling properties. The long-term stability of hydroxyapatite and the solubility of β-TCP provide an osteoconductive graft with an optimal resorption profile. Interconnected macropores provide a porous, osteoconductive matrix that mimics a natural scaffold for cellular ingrowth and revascularization. Three-dimensional micropores enhance the flow and circulation of biological fluids. We currently market OSTEOMATRIX+ in different preparations (PureBone), bioactive synthetics (Signafusethe United States.

EXTRACTOR is a complementary and Interface),cost-effective solution designed to add needed cells and signals to aid in bone healing. EXTRACTOR provides a collagen ceramic matrix (OsteoMatrix)six-ported cannula with a simplistic design for more flexible positioning and twoenhanced marrow extraction. The large side port design of EXTRACTOR allows for better access and retrieval of the bone marrow isolation systems (CellXtractaspirate which contains the cells and Extractor)signals needed for solid bone formation. The “twin peaks” tip design allows for easy insertion through the hard wall of the cortical bone. An ergonomically designed handle allows the clinician to apply consistent pressure for greater control. We currently market EXTRACTOR in the United States.

Reficio Demineralized Bone Matrix (“Reficio DBM”) is a putty comprised of human demineralized bone matrix and a biocompatible bioabsorbable carrier, carboxymethylcellulose, mixed into a putty-like consistency for ease in surgical use. Reficio DBM is indicated for use as a bone void filler and bone graft substitute for voids or gaps that are not intrinsic to the stability to the bony structure, specifically for the treatment of surgically created osseous defects or osseous defects from traumatic injury to the bone. Reficio DBM can be used for extremities, posterolateral spine and pelvis.

The neXus Ultrasonic Surgical System (“neXus”) is a next generation integrated ultrasonic surgical platform that combines all the features of our existing Surgical Solutions applications, including BoneScalpel®, BoneScalpel Access™, SonicOne and SonaStar into a single fully integrated system, setting a foundation for future developments to fulfill unmet customer needs. The neXus platform is driven by a proprietary digital algorithm designed to provide more power, efficiency, and control for the surgeon. The device incorporates technology that allows for intuitive set-up and use. The neXus system allows for safe and efficient resection of hard and soft tissue, limiting collateral damage to adjacent tissue as compared to conventional surgical instruments, and can be used in a variety of different surgical specialties. In addition, neXus provides users a simple and intuitive system enabled via a digital touchscreen display and smart system set-up across all applications. This allows a hospital to access all of our Surgical Solutions product offerings on this all-in-one console. The neXus Ultrasonic Surgical System has been commercialized successfully in several global markets.


The BoneScalpel® is a state of the art, surgical solution enabling precise cuts in hard tissue (e.g., bone). The device allows for the preservation of surrounding soft tissue structures because of its mechanism-of-action, which is micro-reciprocating movements. This device enables precise linear or curved cuts, on any plane, with precision not normally associated with powered instrumentation. We believe that BoneScalpel offers the speed and convenience of a powered instrument without the dangers associated with conventional rotary devices. The effect on surrounding soft tissue is limited due to the elastic and flexible structure of healthy tissue. We believe this is a significant advantage in anatomical regions like the spine where patient safety is of primary concern. In addition, the linear motion of the blunt, tissue-impacting tips avoids accidental ‘trapping’ of soft tissue while largely eliminating the high-speed spinning and tearing associated with rotary power instruments. We believe the BoneScalpel allows surgeons to improve on existing surgical techniques by creating new approaches to bone cutting, sculpting, and removal, leading to substantial time-savings and increased operation efficiencies.
In addition to BoneScalpel, the Company received 510(k) clearance for its neXus® BoneScalpel® Access™ system in December 2021. Specifically, the BoneScalpel Access handpiece and its accessories provide surgeons with a new option for confined spaces during minimally invasive surgery, enabling safe and powerful bone removal with maximum visualization. In addition, BoneScalpel Access allows for en-bloc resection and the shaving and sculpting of bone, with built-in irrigation and aspiration with improved ergonomics for the end user. The BoneScalpel Access handpiece represents best-in-class among ultrasonic surgical platforms, and surgeon feedback following the U.S. market roll out has been positive.


The SonaStar System provides powerful and precise ablation and removal of soft tissue. The SonaStar has been used for a wide variety of surgical procedures applying both open and minimally invasive approaches, including neurosurgery and general surgery. In addition to soft tissue applications, SonaStar may be used with hard tissue tips to enable precise shaping or shaving of bony structures that prevent access to partially or completely hidden soft tissue masses.
In addition to SonaStar, Bioventus received FDA 510(k) approval in July 2022 for its neXus® SonaStar Elite® handpiece and accessories, which expanded the frequency capabilities of the neXus System, adding 36 kHz. While the neXus system can be used in many clinical applications including neurosurgery, the SonaStar Elite handpiece has been cleared for resection of tumors with varying consistencies ranging from soft to firm, including the removal of brain and spinal tumors. The SonaStar Elite handpiece represents the latest innovation in the neXus ultrasonic surgical pipeline.

The SonicOne Ultrasonic Cleansing and Debridement System is a highly innovative, tissue specific approach for the removal of devitalized or necrotic tissue and fibrin deposits while sparing viable, surrounding cellular structures. The tissue specific capability is, in part, due to healthy and viable tissue structures’ higher elasticity and flexibility than necrotic tissue and resistance to destruction from the impact effects of ultrasound. The ultrasonic debridement process separates devitalized tissue from viable tissue layers, allowing for a more defined treatment and, usually, a reduced pain sensation. We believe that SonicOne establishes a new standard in wound bed preparation, the essential first step in the healing process, while contributing to faster patient healing.
Developmental and clinical pipeline for Surgical Solutions (including Investments)
As we build the body of clinical evidence supporting our products, we continue to look for and execute on opportunities to innovate in our BGSSurgical Solutions portfolio. To meet growing market demand and evolving surgical techniques,specifically the needs of surgeons, we continue to develop product extensions on our surgical technology platforms, including the OsteoAmp and adjust formulations. For example, we launched OsteoAMP Select in 2019neXus platform.
Restorative Therapies
Our Restorative Therapies product portfolio consists of an ultrasonic bone stimulation system and we expecta portfolio of products comprised of Advanced Rehabilitation devices designed to launch OsteoAMP Flowable in 2021. We designed OsteoAMP Flowablehelp patients regain leg or hand function due to be moldable and easy to use, with a convenient, ready to use syringe.stroke, multiple sclerosis or other central nervous system disorders.
| | | | | | | | | | | | | | | | | | | | |
Product | | Indications | | Description | | Regulatory pathway / year launched |
Allograft |
| | | | | | |
| | Orthopedic, neurosurgical and reconstructive bone grafting procedures | | An allogeneic bone graft that is available in
multiple formats (fibers, putty, sponge and
granules) that is processed with bone
marrow cells to maintain the wide array of
growth factors present in native bone | | •Section 361 HCT/P / 2009
|
| | | | | | |
| | Posterolateral spine procedures | | Derived from human allograft bone tissue
and is combined with a migration-resistant
resorbable carrier and formulated into a
putty | | •510(k) / 2012
|
| | | | | | |
| | Orthopedic, neurosurgical and reconstructive bone grafting procedures | | 100% cancellous bone with compressible,
elastic and sponge-like attributes, offered in
filler, block and strip options, as well as
mineralized chips | | •Section 361 HCT/P / 2012
|
| | | | | | |
Synthetic | | | | | | |
| | | | | | |
| | Standalone posterolateral spine, extremities and pelvis, as well as a bone graft extender in the posterolateral spine | | Bioactive synthetic bone graft substitute
comprised of a mixture of calcium
phosphate granules and bioglass granules
suspended in a resorbable polymer carrier
that facilitates handling and delivery of the
granule components to fill spaces of
missing bone | | •510(k) / 2014
|
| | | | | | |
| | Posterolateral spine when mixed with autograft, extremities and pelvis | | Bioactive synthetic bone graft in the form
of irregular granules of bioglass to repair
bone defects | | •510(k) / 2011
|
| | | | | | |
| | Posterolateral spine, extremities and pelvis | | Next-generation mineralized two-phase
calcium phosphate bone void filler
comprised of a collagen scaffold designed
for optimized intra-operative handling and
biologic responsiveness at the defect site | | •510(k) / 2010
|
| | | | | | |
| | Posterolateral spine, extremities and pelvis | | Next-generation mineralized bone void filler
comprised of bioglass and biphasic mineral
granules embedded in a collagen scaffold
designed for optimized intra-operative
handling and biologic responsiveness at the
defect site. | | •510(k) / 2020
|

Minimally Invasive Fracture Treatment
BoneEXOGEN® is an ultrasound bone stimulation system for the non-invasive treatment of established nonunion fractures
Fractures, also known as broken bones, occur when there is a high force or impact put on a bone, most commonly from trauma resulting from sports injuries, car accidents, falls or from osteoporosis, which is bone weakening due to aging. Immediately following a fracture, patients are treated to realign the fractured bone ends. If possible or required, the affected limb is immobilized using plaster or a splint. In some cases, fractures require surgical fixation with devices such as screws, plates, rods and frames. X-rays, CT and MRI imaging are utilized to verify alignment of the bone and to assess progress towards healing.
A fracture is considered acertain fresh fracture during the first 14 days after the fracture occurs. After a fracture is treated, new bone tissue begins to form and bridge the gap. With modern treatment methods, most fractures heal spontaneously over the course of several months following injury. However, some fractures fail to heal even when they receive the best surgical or non-surgical treatments. This condition may be diagnosed as a nonunion fracture. Nonunions may occur due to mechanical instability of the fracture site, due to inadequate immobilization, poor blood supply, gaps in bone to bone contact, or a number of comorbidities experienced by the patient. In clinical literature, it is estimated that five to ten percent of all fractures fail to heal, often in high impact fractures or in patients that have compromised health from old age, obesity, cardiovascular disorders, arthritis, diabetes or smoking.fractures. A nonunion fracture is considered to be established when the fracture site shows no visibly progressive signs of healing. Nonunions have a negative impact on quality of life with symptoms, such as reduced mobility, swelling, pain, tenderness, deformity and difficulty bearing weight. Patients with nonunions may undergo surgery when certain conditions occur, such as an unstable or misaligned fracture, or a larger inter-fragment gap. Some nonunions can be treated non-surgically using bone stimulation devices.
Long bone stimulation systems
We offer our Exogen ultrasound bone healing system for the non-invasive treatment of established nonunion fractures and certain fresh fractures. Our Exogen system was the number one prescribed device in the long bone growth stimulatory market in 2018. ItEXOGEN has been sold commercially for over 25 years and is the only FDA-approved device on the market for the accelerated healing of fresh, closed posteriorly displaced distal fractures of the radius and fresh, closed or gradeGrade I open long bone fractures.
| | | | | | | | | | | | | | | | | | | | |
Product | | Description | | Regulatory pathway | | Region where marketed(1)
|
| | | | | | |
| | Ultrasound bone healing system for nonunion fractures and fresh fractures to the tibia and radius(2)
| | •PMA
•Device approval by Health Canada
•CE mark and other registrations(2)
| | •United States
•Canada
•Europe
•Japan
|
____________________
(1)Our Exogen system EXOGEN utilizes low-intensity pulsed ultrasound technology to stimulate the body’s natural bone stimulation process. EXOGEN is used to administer treatment in a location of convenience with an easy to use interface that tracks treatment use and promotes compliance. EXOGEN is indicated in the United States for the non-invasive treatment of established nonunions,nonunion fractures excluding skull and vertebra fractures, and for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open long bone fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization. We own our Exogen system and market it bothEXOGEN is marketed in and outside the United States.
(2)Exogen is alsoStates, Canada, Europe and Japan, and approved for marketing in Australia, Japan, New Zealand, Saudi Arabia, Turkey and the UAE.
Our Exogen
L300 GO is a functional electrical stimulation that produces measurable mobility improvements for patients living with foot drop and thigh weakness. A 3-axis gyroscope and accelerometer are embedded in the Stimulator to monitor user movement in all three kinematic planes and deploy stimulation in 0.01 seconds of detecting a valid gait event. Through an adaptive, learning algorithm, L300 Go is designed to detect gait events, providing stimulation precisely when needed making it easier for users to clear their foot at different walking speeds, on stairs, ramps, and while navigating uneven terrain.

H200 Wireless is a hand rehabilitation system is used to administer treatmentthat supports the wrist in a locationfunctioning position, allowing the fingers and thumb to move efficiently while reaching, grasping and pinching. H200 Wireless has two main parts that communicate wirelessly with each other: the functional stimulation support (“orthosis”) and the control unit (“microprocessor”). These are designed to increase hand function, increase or maintain hand range of convenience, including at home motion, reduce muscle spasms, prevent muscle loss, reeducate muscles and/or work, once daily, for 20 minutes, or as prescribedincrease blood circulation. H200 Wireless is programmed by the patient’s physician, for accelerating bone healing. This therapy provides a cost-effective treatment alternative to surgical intervention for nonunions.
Our Exogen system consists of the portable device, a charger, a gel bottle and strap. The device features a transducer at the end of a coiled cord, a color screen, a power button and a mini-USB charging port to allow for recharging the battery. The transducer sends specifically-programmed low-intensity pulsed ultrasound to the fracture site through the skin and soft tissue, with little or no sensation felt by the patient during the treatment. The gel facilitates ultrasound signal transmission through the patient’s skin. Our Exogen system provides an easy to use interface that tracks treatment use and promotes compliance. In a clinical study of our Exogen system, we observed a 91% patient compliance with the treatment regimen, based on median total time of device usage. An additional support tool for the patient is Exogen Connects, a free smartphone app that provides daily automated treatment reminders and helpful healing information.
Our Exogen system utilizes low-intensity pulsed ultrasound technologyclinician to stimulate the body’s natural bone healing process. The ultrasound output intensityappropriate nerves and muscles of the deviceforearm and hand. We believe this helps to re-educate electrical brain signals, stimulating weak or paralyzed muscles.

Vector is comparablea body weight support system designed to diagnostic ultrasound intensity levels usedaccelerate physical rehabilitation of patients with severe gait and/or balance impairment. The system unloads a programmed amount of weight to enable the patient to practice walking with less than his or her full body weight. Vector is designed to alleviate the risk of falling and provides a feeling of security, instilling confidence in obstetrical sonogram procedurespatients and empowering clinicians to develop effective and challenging rehabilitation regimens. Vector is designed to reduce safety risks so clinicians can remain focused on their patient’s execution of an activity. Designed for fetal monitoringboth physical and occupational therapy, Vector is typically only onedesigned to five percent of the output intensity of conventional therapeutic ultrasound devices usedprovide a safe environment and real-world experience for physical therapy. Someadult and pediatric patients report experiencing a tingling sensation at the treatment site. The depthrecovering from stroke, amputations, and breadth of the Exogen ultrasound signal enables it to treat superficialorthopedic, brain and deep indicated fractures. Exogen ultrasound is osteoinductive, which means it stimulates cells to differentiate into osteoblasts, or cells that make new bone. The growth of this new bone helps bridge the gap at the fracture site.spinal cord injuries.
Comparison
The Bioness Integrated Therapy System® (“BITS”) is an affordable and versatile solution for vision, motor and balance training for individuals, including those with deficits resulting from traumatic injuries and movement disorders as well as competitive athletes. BITS is a multi-disciplinary therapy solution designed to motivate patients and enhance clinician efficiency. BITS’s interactive touchscreen and diverse program options challenge patients to improve performance through the use of U.S. long bone stimulation devices
| | | | | | | | | | | | | | | | | | | | |
Product
Manufacturer
| | Daily treatment
times | | Technology | | Indications |
| | | | | | |
Bioventus
| | 20 minutes | | Low-intensity pulsed ultrasound | | Nonunions and select fresh fractures(2)
|
| | | | | | |
CMF OL1000
DJO Global, Inc.
| | 30 minutes | | Combined magnetic field | | Nonunions |
| | | | | | |
Physio-Stim
Orthofix International B.V.
| | 3 hours | | Pulsed electromagnetic field | | Nonunions |
| | | | | | |
EBI Bone Healing System
Zimmer Biomet Holdings, Inc.
| | 10 hours | | Pulsed electromagnetic field | | Nonunions |
| | | | | | |
OsteoGen
Zimmer Biomet Holdings, Inc.
| | 24 hours | | Direct electrical current (implanted) | | Nonunions |
| | | | | | |
Orthopak 2 Bone Growth Stimulator
Zimmer Biomet Holdings, Inc.
| | 24 hours | | Capacitive coupling | | Nonunions |
____________________
(1)Heal ratesvisual motor activities, visual and auditory processing, cognitive skills, endurance and balance training. Standardized assessments and progress reports make documenting outcomes quick and easy. With the large variety of BITS programs, therapists can choose activities that are tailored to each individual. BITS programs can be further modified to accommodate varying degrees of difficulties. With hundreds of possible parameter combinations, BITS can be customized even further to provide a unique therapy experience for fresh fracture as compared to placebo.
(2)Our Exogen systemeach patient. BITS is indicated in the United Statesoptimized for the non-invasive treatment of established nonunions excluding skulloccupational therapy, physical therapy and vertebra and for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open long bone fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
Exogen clinical data
In a meta-analysis published by Leighton et al. in 2017 studied heal rates of many different fracture nonunions treated with our Exogen system. A total of 13 eligible studies were identified which reported success of treatment with our Exogen system in 1,441 nonunions. Overall, that analysis estimated that 82% of nonunions of at least three months in age treated with our Exogen system successfully healed. Because healing of established nonunion is not expected without treatment, these findings are compelling. Our Exogen system may be most useful in patients for whom surgery is high risk. With an observed overall average nonunion heal rate of at least 80% after treatment with our Exogen system, the study authors concluded that treatment with our Exogen system was comparable to surgical treatment for nonunion.
Additional published evidence supports the efficacy of our Exogen system in treating fracture nonunions. Established fracture nonunions rarely heal without corrective surgery, though nonunion revision surgery is expensive, invasive and the expected heal rate averages approximately 86%. In a study that looked at patient data collected over a four-year period, Zura et al. found that was that the Exogen system enhanced the heal rate among chronic nonunions and even healed fractures that had been nonunion for more than 10 years, without further surgical intervention. Heal rate was 86.2% among patients with fractures that had not healed for at least one year, 82.7% among 98 patients with chronic nonunion of greater than five years duration, and furthermore 12 patients healed after chronic nonunion of greater than 10 years. Therefore, our Exogen system offers a heal rate comparable to surgery, with fewer associated risks and morbidities.speech language pathology.
Developmental and clinical pipeline
Ongoing Bioventus-sponsored clinical studies (B.O.N.E.S.)
While currently indicated for the treatment of both established nonunions, excluding skull and vertebrae, and certain types of conservatively managed acute fractures of the tibia and radius, our Exogen system’s use in fracture care management has grown over its 20 year clinical history in both the United States and internationally. The use of our Exogen system for the management of fresh fractures has been the subject of numerous published peer reviewed research articles. The current prescription data indicate that the product’s use in routine practice of fresh fracture management is based on clinician’s determination of medical necessity, in an effort to mitigate risk of progression to fracture nonunion in at-risk patients.
In order to quantify the effectiveness of our Exogen system in mitigating the risk of progression to fracture nonunion, and in an effort to obtain regulatory approval for expanded indications, we are seeking to supplement the body of clinical knowledge in an innovative population-based clinical development program, B.O.N.E.S., which stands for Bioventus Observational Non-interventional Exogen Studies. With enrollment started in late 2017, the B.O.N.E.S. clinical study design includes the parallel conduct of three independent study protocols which, taken together, are designed to prospectively include more than 3,000 Exogen-treated patients presenting with certain risk factors to be observed over the course of 9 to 12 months. Our Exogen system treated patients will be propensity matched to one or more untreated controls extracted from a real-world health claims database provided by Truven Healthcare Analytics, generating a total sample size of at least 6,000 patients. The program involves the concurrent execution of three studies on pre-specified anatomical locations, such as the tibia, scaphoid and fifth metatarsal, with the objective of determining if the use of our Exogen system mitigates risk of fracture nonunion in predisposed patients. Depending on the results from our studies, we plan to submit a total of three PMA supplements to the FDA, the first of which was submitted in December 2020 seeking approval for the adjunctive treatment of acute and delayed metatarsal fractures to reduce the risk of non-union. We plan to submit the second PMA supplement in the second quarter of 2022 and the third PMA supplement in either the third or fourth quarter of 2023.
Sales and marketingRestorative Therapies
Our expansive direct sales and distribution channel across our product portfolio provides us with broad and differentiated customer reach and allows us to serve physicians spanning the orthopedic continuum, including sports medicine, total joint reconstruction, hand and upper extremities,extremity, foot and ankle, and podiatric surgery, trauma, spine and neurosurgery. OurWe believe our products or procedures using our products are widely reimbursed by both public and private health insurers and are sold in the physician’s office or clinic, ASCs, and in the hospital setting in the United States and across 37approximately 52 other countries. Our sales team and distributors work directly with our physician customers on a frequent basis, providing us with a significant opportunity to introduce new products and upsellbasis.
Product revenue
Products from our current portfolio. We believe our sales organization will provide us with an opportunity to efficiently roll out our deep pipelinePain Treatments, Restorative Therapies and participate in business development opportunities going forward.
Our OA joint pain treatment and joint preservation products and our minimally invasive fracture treatment productsSurgical Solutions groups are sold by a direct sales team of approximately 240teams in the United States and approximately 45 internationally as of December 31, 2020. This directa complementary indirect sales team for Surgical Solutions. That team is complimentedsupported by approximately 20 account representatives who worka broad management team in addition to a market access team focused on expanding approvals with our sales team to provide account access through IDNs, GPOs and payer contracting. Our direct sales organization, totaling approximately 305 globally, is supplemented by approximately 35 sales managers. Our sales leaders have considerable experience, with an average of five years of experience. Our BGSpayers. Internationally our products are sold by approximately 170 independent distributors in the United States, each with their own independentthrough a mix of direct and indirect sales force, supported by our 15 member regionalized sales support team as of December 31, 2020. We market our BGSs primarily to orthopedic spine surgeonsteams and neurosurgeons for use in the operating room in both the hospital and ASC setting. We believe that our broad customer reach has and will continue to enable strong and durable growth in each of our verticals and provides a significant foundation for future product launches.distributors. We support our entire sales organization with extensive training to help them excel, and we have a performance culture built on serving our core orthopedic patient customers and delivering our products to a variety of physicians and care settings.
Research and clinical operations
We see significant opportunity to develop innovative and clinically differentiated products internally with our experienced research and development team. We are focused on internal research and development to broaden our portfolio of therapies to manage OA joint pain and joint preservation, expand our Exogen system product label and undertake clinical research to support commercialization of our next-generation of BGS products.
As a result, we expect our research and development expense to increase to the mid-single digits as a percentage of net sales as we introduce new products, extend existing product lines and expand indications. Our research and development activities are focused on product development in BGSs, treatments for OA and soft tissue surgery. Our clinical research is focused on running the B.O.N.E.S. and MOTYS clinical programs, as well as continuing to build our body of clinical evidence to demonstrate the efficacy and value of our products through collaborations with prominent academic investigators. We collaborate with academic centers of excellence, leading contract research organizations and other industrial groups to complement and expedite execution of our research and development programs and minimize fixed costs. Research and development expense, including spending on our clinical evidence development efforts, totaled $11.2 million, $11.1 million and $8.1 million for the years ended December 31, 2020, 2019 and 2018, respectively.
Competition
The medical device industry is highly competitive, subject to change and significantly affected by the activities of industry participants. We believe that the principal competitive factors in our markets are product features, value-added solutions, reliability, clinical and economic evidence, reimbursement coverage, and price. Customer support, reputation, and efficient distribution are also important factors. The multi-injection HA viscosupplementation therapiesspeed with which we can develop products, complete clinical testing and regulatory clearance processes and supply commercial quantities of our products to the market are therefore important competitive factors. We compete with many companies having more significant capital resources, larger research laboratories and more extensive distribution systems than we do.
Our Pain Treatments that we own or distribute compete againstwith products from Ferring Pharmaceutical Inc.’s Euflexxa,, Fidia Farmaceutici S.p.A.’s Hyalgan,, DePuy Orthopaedics, Inc. (Johnson & Johnson’s) Orthovisc,Johnson), and Sanofi S.A.’s SynviscS.A, OrthogenRx Inc. (Avanos) and OrthogenRx Inc.’s GenVisc 850. Thesefor peripheral nerve stimulation specifically we compete with SPR Therapeutics, Nalu and Stimwave.
Our Surgical Solution products have faced significant competition from single injection therapies, such as Sanofi S.A.’s Synvisc-One, Zimmer Biomet Holdings, Inc.’s Gel-One and DePuy Orthopaedics, Inc. (Johnson & Johnson’s) Monovisc. Our BGS product portfolio competescompete with products from Medtronic, DePuy Orthopaedics, Inc. (Johnson & Johnson), Stryker Corporation, NuVasive, Inc., SeaSpine, Inc., Orthofix Medical Inc., Zimmer Biomet Holdings, Inc. and Globus Medical Inc., Johnson & Johnson, Integra Life Sciences, Inc., and Söering.
Our Exogen system competesRestorative Therapies compete with products marketed by Orthofix Medical Inc., Zimmer Biomet Holdings, Inc., Enovis, MiMedx, Hanger Orthopedics, XFT Medical, Rewalk Robotics, Ekso Bionics, Aretech LLC and DJO Global Inc.
At any time, these or other market participants may develop alternative treatments, products or procedures that compete directly or indirectly with our products. They may also develop and patent processes or products earlier than we can or obtain regulatory clearance or approvals for competing products more rapidly than we can.
Intellectual propertyDIH Medical.
We strive to protect and enhance the proprietary technologies, inventions and improvements that we believe are important to our business, including seeking, maintaining and defending patent rights, whether developed internally or licensed from third parties.third-parties. Our policy is to seek to protect our proprietary position by, among other methods, pursuing and obtaining patent protection in the United States and in jurisdictions outside of the United States related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trademarks, trade secrets and careful monitoring of and contractual obligations with respect to our proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
Patents, trade secrets, assignments and licenses
We rely on our abilitya combination of patents, trade secrets, assignment and license agreements, and non-disclosure agreements to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business, defend and enforce our patents, maintain our licenses to use intellectual property owned by third parties and operate without infringing the valid and enforceable patents and other proprietary rights of third parties. For important factors related toprotect our proprietary technology, inventions and improvements, please see the section entitled Part I, Item 1A. Risk Factors—Risks related to intellectual property.
Patents
We own numerous patents and/or patent applications which relate to our material products, including patents and/or patent applications with respect to our Exogen system, OsteoAMP and MOTYS.products. Although in the aggregate our intellectual property is of material importance to our business, we do not believe that any single patent is of material importance to our product portfolio. As of November 13, 2020,December 31, 2023, we owned four118 issued U.S. patents and twosix pending U.S. patent applications relating to our material products. We also owned nine174 issued foreign patents and 1139 pending foreign patent applications directed to our material products. Our patents and patent applications as of November 30, 2020December 31, 2023 directed to our material products are summarized below.
We ownedown three issued U.S. patents and one issued foreign patent in Australia directed to our Exogen system. The U.S. patents are expected to expire between 2025 and 2029, and the foreign patent is expected to expire in 2025.
We owned oneown two issued U.S. patent, one pending U.S. patent application, eightpatents, ten issued foreign patents, and teneight pending foreign patent applications directed to our OsteoAMP product, including foreign patents and patent applications in Europe, Asia, Canada and Australia. The issued U.S. patent is expected to expire in 2029. The issued foreign patents are expected to expire in 2029. The pending patent applications, if issued, are expected to expire in 2029, without accounting for potential patent term extensions and adjustments.
We also own ten issued U.S. patents and twelve issued foreign patents in Australia, Canada, Europe, and Japan directed to our StimRouter system. The U.S. patents are expected to expire between 2026 and 2031, and the foreign patents are expected to expire between 2028 and 2030.
We also own twenty-three issued U.S. patents, two pending U.S. patent applications, fifty-seven issued foreign patents, and three pending foreign patent applications directed to our L300 system, including foreign patents and patent applications in Australia, Canada, Europe, and Japan. The U.S. patents are expected to expire between 2026 and 2037, and the foreign patents are expected to expire between 2026 and 2037. The pending patent applications, if issued, are expected to expire between 2032 and 2037, without accounting for potential patent term extensions and adjustments.
We also own fifteen issued U.S. patents, three pending U.S. patent applications, seventeen issued foreign patents, and five pending foreign patent applications directed to our Vector Gait and Safety System, including foreign patents and patent applications in in Australia, Canada, Europe, and Japan. The U.S. patents are expected to expire between 2033 and 2038, and the foreign patents are expected to expire between 2034 and 2037. The pending patent applications, if issued, are expected to expire between 2033 and 2038, without accounting for potential patent term extensions and adjustments.
We also own one issued U.S. patent, one issued foreign patent in Australia and one issued foreign patent in Canada directed to our Bioness Integrated Therapy System (“BITS”). The U.S. patent is expected to expire in 2037, and the foreign patents are expected to expire in 2036.
We also own two issued U.S. patents and one pending U.S. patent application and onefour pending Patent Cooperation Treaty applicationforeign patent applications directed to MOTYS. Patents issuing from theseour TalisMann product, including foreign patent applications if any,in Australia, Canada, Europe, and Japan. The U.S. patents are expected to expire inbetween 2039 and 2040. The pending patent applications, if issued, are expected to expire between 2039 and 2040, without accounting for potential patent term extensions and adjustments. We also own twenty-nine issued U.S. patents, thirty-one issued foreign patents and seven pending foreign patents directed to our BoneScalpel product.
Our patents and pending patent applications directed to our material products are further detailed in the below table.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Country | | Application
Number | | Filing Date | | Patent Number | | Application
Status | | Expected
Expiration
Date | | Description | | Product |
| AU | | 2009324417 | | December 13, 2009 | | 2009324417 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| AU | | 2014259553 | | November 14, 2014 | | 2014259553 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Country | | Application
Number | | Filing Date | | Patent Number | | Application
Status | | Expected
Expiration
Date | | Description | | Product |
| AU | | 2016213839 | | August 11, 2016 | | 2016213839 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| CA | | 2746668 | | December 13, 2009 | | 2746668 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| CN | | 200980155596.X | | December 13, 2009 | | 200980155596.X | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| CN | | 201410413348.3 | | August 20, 2014 | | 201410413348.3 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| EP | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| HK | | 15105678.1 | | June 16, 2015 | | HK1205007 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| IN | | 2567/KOLNP/2011 | | February 4, 2016 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| KR | | 10-2011-7016270 | | July 2, 2019 | | 10-1713346 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| US | | 15/016072 | | December 13, 2009 | | 10383974 | | Issued | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| US | | 16/459778 | | February 4, 2016 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| GB | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| FR | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| DE | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| IT | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| CH | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| BE | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| ES | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| PT | | 9832666.3 | | December 13, 2009 | | | | Pending | | December 2029 | | Directed to methods of making osteoinductive implants | | OsteoAMP |
| US | | 09/925,193 | | August 9, 2001 | | 7429248 | | Granted | | July 2025 | | Directed to applying ultrasound to tissue using a modal converter having a plurality of angled sides. | | Exogen |
| AU | | 2006203281 | | August 1, 2006 | | 2006203281 | | Granted | | August 2025 | | Directed to treating a neuropathy disease with ultrasound using a specific frequency and pulse rate for the signal | | Exogen |
| US | | 11/462271 | | August 3, 2006 | | 8048006 | | Granted | | February 2029 | | Directed to treating a neuropathy disease with ultrasound using a specific frequency and pulse rate for the signal | | Exogen |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Country | | Application
Number | | Filing Date | | Patent Number | | Application
Status | | Expected
Expiration
Date | | Description | | Product |
US | | 12/296,333 | | April 7, 2007 | | 8226582 | | Granted | | June 2028 | | Directed to applying ultrasound to tissue using a modal converter having an oblique angle and speed of sound similar to human tissue | | Exogen |
US | | 17/097,350 | | November 13, 2020 | | | | Pending | | November 2040 | | Directed to placental tissue particulates compositions, methods of treating musculoskeletal or orthopedic conditions, methods of treating pain associated with osteoarthritis, kits and methods of making the compositions | | MOTYS |
PCT | | PCT/US20/60393 | | November 13, 2020 | | | | Pending | | November 2010 | | Directed to placental tissue particulates compositions, for use in treating musculoskeletal or orthopedic conditions, methods of treating pain associated with osteoarthritis, kits and methods of making the compositions | | MOTYS |
Exhibit 99.1 to this Annual Report.Trademarks
We own registered trademarks for Bioventus, Bioness, BITS, Bonescalpel, BoneScalpel Access, Cellxtract, Durolane, Exogen, Exponent, Gelsyn-3, LiveOn, L300 Go, Misonix, Ness, Ness L300, neXus, OsteoAMP, Osteofuse, Prohesion, PureBone, SAFHS, Signafuse, Sonastar, SonaStar Elite, StimRouter and Signafusethe Vector Gait and Safety System in the United States.
Trade secrets
We may rely on trade secret law to protect some of our technology, including the processing of tissue for OsteoAMP.technology. Trade secrets, however, can be difficult to protect. We seek to protect our proprietary technology and manufacturing process, in part, by confidentiality and invention assignment agreements with employees, consultants scientific advisors and contractors, under which they are bound to assign to us certain inventions that are made during the termcourse of their employmentperforming work for us and relate to our business, unless there is an exception.business. These agreements further restrict the use and disclosure of Bioventus’ confidential information and proprietary information belonging to any third party. These agreements further prohibit our employees from using, disclosing, or bringing onto the premises any proprietary information belonging to any third-party. In addition, our consultants, scientific advisors and contractors are required to sign agreements under which they must assign to us any inventions that relate to our business. These agreements also prohibit these third-parties from incorporating into any inventions the proprietary rightsthird party.
We also seek to preserve the integrity and confidentiality of our data and trade secrets by taking commercially reasonable efforts to maintain the physical security of our premises and physical and electronic security of our information technology systems.
While we have confidence in these individuals, organizations and systems, our security measures may be breached, or may otherwise prove inadequate to protect the integrity and confidentiality of our data and trade secrets. Further, our agreements may be breached (or not obtained in the first place) and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
In addition to patents, trademarks, and trade secrets, we also rely on assignment and license agreements, pursuant to which we may license rights under patents held by third parties, and non-disclosure agreements, to protect our proprietary intellectual property.
We obtain assignments or licenses of varying durations for certain of our products from third parties. We typically acquire rights under such assignments or licenses in exchange for lump-sum payments or arrangements under which we pay a percentage of sales to the licensor. However, while assignments or licenses to us generallysuch rights are irrevocable, no assurance can be given that these arrangements will continue to be made available to us on financial terms that are acceptable to us, or at all. The terms of our license and assignment agreements vary in length from a specified number of years to the life of product patents or the economic life of the product. These agreements generally provide for royalty payments and termination rights in the event of a material breach.
We will continue to seek patent, trademark, and copyright protection as we deem advisable to protect the markets for our products and to support our research and development efforts.Manufacturing and supply
Our HA viscosupplementation therapiesWe largely manufacture and certain ofassemble our surgicalmedical device products are manufactured exclusively by single-source third-party manufacturers, pursuant to multi-year supply agreements. We work closely with each of our manufacturing partners and provide them with a forecast, which enables them to better capacity plan and sequence their production efficiently.
For Durolane, we are subject to minimum order volumes for each order and purchase amounts are also based in part on forecasts. For GELSYN-3, we will be subject to certain annual minimum purchase requirements and purchase amounts based on rolling forecasts. For SUPARTZ FX, we are subject to certain annual minimum purchase requirements based on a percentage of our SUPARTZ FX annual forecast.
For Durolane, in December 2016, we entered into an amended and restated supply agreement, or the Q-Med Supply Agreement, with Q-Med AB, or Q-Med. Under the Q-Med Supply Agreement, Q-Med supplies Durolane products exclusively to us for sale in the United States for use in the in the prevention or treatment of pain due to OA on a purchase order basis, based on the amounts of Durolane we require as set forth in rolling forecasts and we are subject to certain semi-annual minimum purchase requirements based on a percentage of our Durolane forecast.
We assemble, inspect, test and package our Exogen system at our facilityproduction facilities located in Cordova, Tennessee with components supplied by third-party suppliers. Our Exogen system includes a transducer which is a key component that is supplied by a single-source supplier. We perform inspections of these components before use in our manufacturing operations.TN, Farmingdale, NY, Valencia, CA and Hod Hasharon, Israel. We believe our manufacturing operations are in compliance with regulations mandated by the FDA. We are an FDA-registered medical device manufacturer. Our manufacturing facilities and processes are subject to periodic inspections and audits by various federal, state and foreign regulatory agencies. Our products include components manufactured by other companies in the United States and elsewhere.
MTF willSome of our products and product components are manufactured exclusively manufactureby single-source third-party manufacturers, pursuant to multi-year supply agreements that may include minimum order volumes. We work closely with each of our manufacturing partners and supply MOTYS under current Good Tissue Practices (cGTPs)provide them with a forecast, which enables them to us to allowbetter capacity plan and sequence their production efficiently.
We may encounter difficulty in obtaining materials, supplies and components adequate for our limited commercialization that began in the fourth quarter of 2020 under the tissue regulations while we pursue a BLA for the product. MTF is responsible for obtaining and storing all materials, including all tissue materials, required for the manufacture, testing, handling, packaging, labeling, release and delivery of the product to us.
anticipated short-term needs. We intend to maintain sufficient supplies of the products and components from these single-source suppliers in the event that one or more of these suppliers were to encounter certain interruptions in supply.supply of products including L360 and H200 both of which are manufactured in Israel.
Government regulation
Government regulation of medical devices
Our medical devicesproducts and operations are subject to extensive regulation by numerous government agencies, including the FDA and comparable foreign agencies. The FDAother federal and other U.S. and foreign governmental agencies regulate, among other things, with respect to medical devices:
•design, development and manufacturing;
•testing, labeling, content and language of instructions for use and storage;
•clinical trials;
•product safety;
•marketing, sales and distribution;
•premarket clearance and approval;
•record keeping procedures;
•advertising and promotion;
•recalls and field safety corrective actions;
•postmarket surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury;
•postmarket approval studies; and
•product import and export.
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
FDA premarket clearance and approval requirements
Each medical device we seek to commercially distributestate authorities in the United States, must first receiveas well as comparable authorities in foreign jurisdictions. In the United States, our products and product candidates are regulated as either medical devices under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and its implementing regulations, or as drugs or biological products under the FDCA and the Public Health Service Act (“PHSA”), and their implementing regulations, each as amended and enforced by the FDA. The FDA regulates the development, design, non-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, adverse event reporting, advertising, promotion, marketing and distribution, and import and export of medical devices and biological products to ensure that such products distributed domestically are safe and effective for their intended uses and otherwise meet the applicable requirements of the FDCA and PHSA.
U.S. Regulation of Medical Devices
In the United States, the majority of our products are regulated as medical devices by the FDA. Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) clearancepremarket notification, or approval of a PMA application fromapplication. Under the FDA, unless specifically exempt. The FDA classifies allFDCA, medical devices are classified into one of three classes. Devices deemed to pose lower risk are categorized as either classes—Class I, Class II or Class II. Class II devices requireIII—depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to submit to the FDA a 510(k) pre-market notification requesting clearance of the device for commercial distribution in the United States. Class I devices are exempted from this requirement.ensure its safety and effectiveness. Devices deemed by the FDA to pose the greatest risk,risks, such as life sustaining, life-supporting, selectedlife supporting or some implantable devices, or devices deemedthat have a new intended use, or use advanced technology that is not substantially equivalent to that of a previously 510(k) clearedlegally marketed device, are categorizedtypically placed into Class III.
While most Class I devices are exempt from the 510(k) premarket notification requirement, manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to a 510(k) premarket notification is generally known as 510(k) clearance. Class III generally requiring submission anddevices require approval of a PMA.premarket approval application, or PMA, evidencing safety and effectiveness of the device.
To obtain 510(k) clearance, wea manufacturer must submit a premarket notification demonstrating to the FDA demonstratingFDA’s satisfaction that the proposed device is “substantially equivalent” to be substantially equivalent to aanother legally marketed device that itself does not require PMA approval (a predicate device.device). A predicate device is a previously cleared 510(k)legally marketed device that is not subject to premarket approval, i.e., a device that was in commercial distribution beforelegally marketed prior to May 28, 1976 (pre-amendment device) and for which the FDA hasa PMA is not yet called for the submission of PMA applications, or isrequired, a device that has been reclassified from Class III to either Class II or I.I, or a device that was found substantially equivalent through the 510(k) process. The standard reviewFDA’s 510(k) clearance process for 510(k)s is between 30 daysusually takes from three to 3twelve months, dependent upon the type of 510(k) filing submitted. Although many 510(k) premarket notifications are cleared without clinical data, in some cases, thebut often takes longer. The FDA may require clinical data to support substantial equivalence. In reviewing a premarket notification, the FDA may request additional information, including clinical data, to make a determination regarding substantial equivalence. In addition, the FDA collects user fees for certain medical device submissions and annual fees for medical device establishments.
If the FDA agrees that the device is substantially equivalent to a lawfully marketed predicate device, it will grant 510(k) clearance to authorize the device for commercialization. If the FDA determines that the device is “not substantially equivalent,” the device is automatically designated as a Class III device. In such cases, the device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the de novo classification process, which may significantly prolong the review processis a route to market for novel medical devices that are low to moderate risk and clearance is never assured.are not substantially equivalent to a predicate device.
After a device receives 510(k) clearance, any subsequent modification of the device that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, requireswill require a new 510(k) clearance or, could requiredepending on the modification, PMA approval or de novo classification. The FDA requires each manufacturer to determine whether the proposed change requires submission and approval of a 510(k), de novo classification request or a PMA application in cases where new indications are sought for which there is no predicate. Non-significant changes are handled via internal documentation by the Company. Each manufacturer must judgefirst instance, but the significance of modifications based on algorithms within FDA 510(k) guidance documents. FDA maycan review any such decision and may disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination not to seek a new 510(k) or other form of marketing authorization for the modification to the 510(k)-cleared product, the FDA maycan require the manufacturer to cease marketing and/or request the recall of the modified device until 510(k) clearance or PMA approval is obtained. We have modified aspectsobtained or a de novo classification is granted.
The PMA process is more demanding than the 510(k) premarket notification process. In a PMA, the manufacturer must demonstrate that the device is safe and effective, and the PMA must be supported by extensive data, including data from preclinical studies and human clinical trials. All clinical investigations of somedevices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption (“IDE”) regulations which govern investigational device labeling, prohibit promotion of our devices since receiving initial regulatory clearance. We concludedthe investigational device, and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. A significant risk device is one that some of those modifications did not significantly affectpresents a potential for serious risk to the health, safety or efficacywelfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. In addition, the study must be approved by, and conducted under the oversight of, an Institutional Review Board (“IRB”), for each clinical site. The IRB is responsible for the initial and continuing review of the IDE, and may impose additional requirements for the conduct of the study. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
In addition to clinical and preclinical data, the PMA must contain a full description of the device and therefore, that new 510(k) clearances were not required. We have also obtained new 510(k) clearances fromits components, a full description of the methods, facilities, and controls used for manufacturing, and proposed labeling. Following receipt of a PMA, the FDA for other modificationsdetermines whether the application is sufficiently complete to our devices. In the future, we may make additional modifications to our products after they have received FDA clearance or approval and in appropriate circumstances, determine that new clearance or approval is unnecessary. However,permit a substantive review. If the FDA may disagree with our determination and ifaccepts the FDA requires usapplication for review, it has 180 days under the FDCA to seek 510(k) clearance or submit newcomplete its review of a PMA, applications for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain the required clearance or approval. Under these circumstances, we may also be subject to significant regulatory fines or other penalties.
We have obtained 510(k) premarket clearance from the FDA for Signafuse Bioactive Bone Graft Putty, Interface Bioactive Bone Graft, Osteomatrix +, and Signafuse Mineralized Collagen Scaffold.
Premarket approval process
A PMA application must be submitted if the medical device isalthough in Class III (although the FDA has the discretion to continue to allow certain pre-amendment Class III devices to use the 510(k) process) or cannot be cleared through the 510(k) process. A PMA application must be supported by, among other things, extensive technical, preclinical, clinical trials, manufacturing and labeling data to demonstrate topractice, the FDA’s satisfaction the safetyreview often takes significantly longer, and effectiveness of the device.
After a PMA application is submitted and filed, the FDA begins an in-depth review of the submitted information. The standard review of such application is six months. During this review period, the FDA may request additional information or clarification of information already provided. This can extend the overall review process and typically PMAs take between oneup to three years in total for approval. Also during the review period, anseveral years. An advisory panel of experts from outside the FDA will usuallymay be convened for a new type of device to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the manufacturing facilityapplicant or its third-party manufacturers’ or suppliers’ facilities to ensure compliance with Quality System Regulation (QSR), which impose elaborate design development, testing, control, documentationthe QSR.
The FDA will approve the new device for commercial distribution if it determines that the data and other quality assurance proceduresinformation in the designPMA constitute valid scientific evidence and manufacturing process.that there is reasonable assurance that the device is safe and effective for its intended use(s). The FDA may approve a PMA application with post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution, and collection of long-term follow-up data from patients in the clinical study that supported approval.PMA approval or requirements to conduct additional clinical studies post-approval. The FDA may condition PMA approval on some form of post-market surveillance when deemed necessary to protect the public health or to provide additional safety and efficacy data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Failure to comply with the conditions of approval can result in materiallymaterial adverse enforcement action, including the loss or withdrawal of the approval. PMA supplements are required for significant modificationsCertain changes to an approved device, such as changes in manufacturing facilities, methods, or quality control procedures, or changes in the manufacturing process, labelingdesign performance specifications, which affect the safety or effectiveness of the product and designdevice, require submission of a device that is approved through the PMA process. PMA supplements requires submission of information needed to support any changes from the device covered by the original PMA and typically do not require clinical data or the convening of an advisory panel. Non-significant changes must be reported to the FDA through an annual report filing with the FDA. In review of this Annual Report, FDA may disagree with a manufacturer’s determination of the level of significance of the change. If the FDA disagrees with a manufacturer’s determination, the FDA may require the manufacturer to cease marketing and/or recall the modified device until PMA supplement, approval is obtained.
Durolane, GELSYN-3, SUPARTZ FX and our Exogen bone healing system have each been approved through the PMA process.
Clinical trials
A clinical trial is typically required to supportor in some cases a PMA and is sometimes required for a 510(k) premarket notification. In the United States, authorization to conduct a clinical trial generally requires submission of an application for an IDE to the FDA. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the investigational protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of patients, unless the product is deemed a non-significant risk device and eligible for abbreviated IDE requirements. Clinical trials for a significant risk device may begin once the IDE application is approved by the FDA as well as the appropriate institutional review boards (IRBs), at the clinical trial sites and the informed consent of the patients participating in the clinical trial is obtained. After a trial begins, the FDA may place it on hold or terminate it if, among other reasons, it concludes that the clinical subjects are exposed to an unacceptable health risk. Any trials we conduct must be conducted in accordance with FDA regulations as well as other federal regulations and state laws concerning human subject protection and privacy. Moreover, the results of a clinical trial may not be sufficient to obtain clearance or approval of the product.
Pervasive and continuing FDA regulationnew PMA.
After a medical device is placed on the market,cleared or approved or otherwise authorized for marketing, numerous FDApervasive regulatory requirements continue to apply unless explicitly exempt. These include:
•establishment registration and device listing with the FDA;
•QSR requirements, which require manufacturers, including but not limited to the following:
•QSRs, which requiresthird-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process;
•Establishment Registration, which requires establishments involved in the productionlabeling and distribution of medical devices, intended for commercial distribution in the United States, to register with the FDA;
•Medical Device Listing, which requires manufacturers to list the devices they have in commercial distribution with the FDA;
•Labelingmarketing regulations, which prohibit “misbranded” devices from entering the market, as well asrequire that promotion is truthful, not misleading, fairly balanced and provide adequate directions for use and that all claims are substantiated, and also prohibit the promotion of products for unapproved or off-label“off-label” uses and impose other restrictions on labeling; FDA guidance on off-label dissemination of information and responding to unsolicited requests for information;
•Postmarket surveillance, including Medical Device Reporting (MDR) requirementsclearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices;
•medical device reporting regulations, which requires manufacturers torequire that a manufacturer report to the FDA if theira device it markets may have caused or contributed to a death or serious injury, or has malfunctioned inand the device or a waysimilar device that it markets would be likely to cause or contribute to a death or serious injury, if itthe malfunction were to recur;
•correction, removal and
•Corrections and Removal Reporting recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the United States Federal Food, Drug, and Cosmetic Act (FDCA)FDCA that may present a risk to health.health;
Table ofcontents•complying with requirements governing Unique Device Identifiers on devices and also requiring the submission of certain information about each device to the FDA’s Global Unique Device Identification Database;
•the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; andThe FDA enforces these requirements by inspection•post-market surveillance activities and market surveillance. Failure to comply with applicable regulatory requirements may result in enforcement action byregulations, which apply when the FDA which may include onedeems them necessary to protect the public health or more ofto provide additional safety and effectiveness data for the following sanctions:device.
•untitled letters or warning letters;HCT/Ps
•fines, injunctions and civil penalties;
•mandatory recall or seizureCertain of our products;
•administrative detention or banning of our products;
•operating restrictions, partial suspension or total shutdown of production;
•refusing our request for 510(k) clearances or PMA approvals for new product versions;
•revocation of 510(k) clearances or PMA approvals previously granted; and
•criminal prosecution and penalties.
U.S. regulation of HCT/Ps
Our products including OsteoAMP and PureBone, are regulated as HCT/P, which is an acronym for human cells, tissuescell, tissue, and cellular and tissue-based products, or HCT/Ps.products. Section 361 of the PHSA authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as “Section 361” HCT/Ps are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, cGTP,current Good Tissue Practices (“cGTPs”) when processing, storing, labeling and distributing HCT/Ps, including required labeling information, stringent record keeping and adverse event reporting, among other applicable requirements and laws. Specifically, cGTPs are requirements that govern the methods used in, and the facilities and controls used for, the manufacture of HCT/Ps in a way that prevents the introduction, transmission, or spread of communicable diseases. Section 361 HCT/Ps do not require 510(k) clearance, PMA approval, BLAs, or other premarket authorization from the FDA before marketing. However, to be regulated as a Section 361 HCT/P, the product must, among other things, be “minimally manipulated,” which for structural tissue products means that the manufacturing processes do not alter the original relevant characteristics of the tissue relating to the tissue’s utility for reconstruction, repair, or replacement and which for cells or nonstructural tissue products, means that the manufacturing processes do not alter the relevant biological characteristics of cells or tissues. A Section 361 HCT/P must also be intended for “homologous use,” which refers to use in the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor.
We believe our OsteoAMP product is properly regulated as a Section 361 HCT/P and therefore have not sought or obtained 510(k) clearance, PMA approval, or licensure through a BLA. However, the FDA’s Center for Devices and Radiological Health (CDRH) issued us a letter in March 2016 in which it asserted that OsteoAMP meets the definition of a medical device and requested that we provide CDRH with information in support of our position that OsteoAMP does not require 510(k) clearance or PMA approval. We provided CDRH with the requested information in support of this position in May 2016 and we have received no further inquiries to date. We believe that CDRH’s assertion is unfounded and inconsistent with a 2011 letter from the FDA concluding that OsteoAMP meets the criteria for regulation solely as a Section 361 HCT/P. However, if the FDA were to disagree and if we are otherwise unsuccessful in asserting our position, the FDA may then require that we obtain 510(k) clearance or PMA approval and that we cease marketing OsteoAMP and/or recall OsteoAMP unless and until we receive clearance or approval. We estimate that if we were to cease marketing OsteoAmp and/or recall OsteoAmp that our net sales would decrease, which would adversely affect our results of operations. See Part I, Item 1A. Risk Factors—Risks related to government regulation—Our HCT/P products are subject to extensive government regulation and our failure to comply with these requirements could cause our business to suffer.
HCT/Ps that do not meet the criteria of Section 361 are regulated under Section 351 of the PHSA. Unlike Section 361 HCT/Ps, HCT/Ps regulated as “351”“Section 351” HCT/Ps are subject to premarket review and/or approval by the FDA, as required.
In November 2017, the FDA released a guidance document entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue—Based Products: Minimal Manipulation and Homologous Use—Guidance for Industry and Food and Drug Administration Staff.” The guidance outlined the FDA’s position that all lyophilized amniotic products are more than minimally manipulated and would therefore require a BLA to be lawfully marketed in the United States. The guidance also indicated that the FDA would exercise enforcement discretion, using a risk-based approach, with respect to the INDInvestigational New Drug ("IND") application and pre-market approval requirements for certain HCT/Ps that had been marketed without marketing authorization, including, among others, lyophilized amniotic products, for a period of 36 months from the issuance date of the guidance to allow manufacturers to pursue its IND application.INDs and/or seek marketing authorizations. Under this approach, the FDA indicated that high-risk products and uses could be subject to immediate enforcement action. In July 2020, the FDA extended its period of enforcement discretion to May 31, 2021.
Failure The FDA resumed enforcement of IND and premarket approval requirements with respect to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including the seizure or recallthese products as of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties.
U.S. regulationRegulation of drugsDrugs and biologicsbiological products
We expect that MOTYS will be regulated byIn the United States, the FDA as a biological product, or biologic,regulates drugs under the FDCA and we plan to submit a BLA to the FDA to allow for the marketing of MOTYS following the expiration of the FDA’s enforcement discretion period for certain HCT/Ps. Biologics are regulatedits implementing regulations, and biologics under both the FDCA and the PHSA and other federal, state, local and foreign statutes andtheir implementing regulations. We, along with third-party contractors, will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our product candidates.
The process required by the FDA before product candidatesa drug or biologic may be marketed in the United States generally involves the following:
•completion of extensive preclinical laboratory tests and animal studies performed in accordance with applicable regulations, including the FDA’s Good Laboratory Practice (GLP), regulations;requirements;
•submission to the FDA of an IND, which must become effective before clinical trials may begin;
•approval by an independent institutional review board, or IRB or ethics committee at each clinical site before the trial is commenced;
•performance of adequate and well-controlled human clinical trials to establish the safety, and effectiveness of the proposed drug candidate and the safety,efficacy, purity and potency of the proposed biologic product candidate for its intended purpose;
•preparation of and submission to the FDA of ana BLA for biologics or New Drug Application (NDA) or BLAfor small molecule drugs after completion of all pivotal clinical trials;
•satisfactory completion of an FDA Advisory Committee review, if applicable;
•a determination by the FDA within 60 days of its receipt of a NDABLA or BLANDA to file the application for review;
•satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with cGMP requirements,cGMPs and to assure that the facilities, methods and controls are adequate to preserve the biological product’s continued safety, purity and effectiveness,potency, and of selected clinical investigation sites to assess compliance with GCPs;Good Clinical Practices (“GCPs”); and
•FDA review and approval of athe BLA or NDA or BLA to permit commercial marketing of the product for particular indications for use in the United States.
Preclinical and Clinical Development
Prior to beginning clinical trials of a new drug or biologic product in the first clinical trial with a product candidate, we must submitUnited States, an IND must be submitted to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol or protocols clinical trials. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology and pharmacodynamic characteristics of the product, chemistry, manufacturing and controls (CMC), information, and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with good clinical practices (GCPs), which include the requirement that all research subjects provide their informed consent for their participation in any clinical study. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site, and must monitor the study until completed. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing preclinical studies and clinical trials and clinical study results to public registries.
For purposes of regulatory approval, human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
•Phase 1. The investigational product is initially introduced into patients with the target disease or condition. These studies are designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness.
•Phase 2. The investigational product is administered to a limited patient population to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks.
•Phase 3. The investigational product is administered to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval.
In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved to gain more information about the product. These so-called Phase 4 studies may be made a condition to approval of the NDA or BLA. Concurrent with clinical trials, companies may complete additional animal studies and develop additional information about the biological characteristics of the product candidate, and must finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product, or for biologics, the safety, purity and potency. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical study investigators. The FDA or the sponsor or its data safety monitoring board may suspend a clinical study at any time on various grounds, including a finding that the research participant or participants are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical study at its institution if the clinical study is not being conducted in accordance with the IRB’s requirements or if the product candidate has been associated with unexpected serious harm to patients. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries. Sponsors of clinical trials of FDA-regulated products may be required to register and disclose certain clinical trial information, which is publicly available at www.clinicaltrials.gov.
NDA or BLA Submission and Review
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of aan NDA or BLA requesting approval to market the product for one or more indications. The NDABLA or BLANDA must include all relevant data available from pertinent preclinical studies and clinical trials,studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s CMCschemistry, manufacturing, controls, and proposed labeling, among other things. The submission of a NDABLA or BLANDA requires payment of a substantial application user fee to the FDA, unless a waiver or exemption applies. The FDA has 60 days from the applicant’s submission of a NDA or BLA to either issue a refusal to file letter or accept the NDA or BLA for filing, indicating that it is sufficiently complete to permit substantive review.
Once a NDA or BLA has been accepted for filing, the FDA’s goal is to review standard applications within ten months after it accepts the application for filing, or, if the application qualifies for priority review, six months after the FDA accepts the application for filing. In both standard and priority reviews, the review process is often significantly extended by FDA requests for additional information or clarification. The FDA reviews a NDA or BLA to determine, among other things, whether a product is safe and effective, or safe, pure, and potent, for its intended use, and whether the facility in which it is manufactured, processed, packed or held meets standards designed to assure and preserve the product’s identity, safety, strength, quality, potency and purity. The FDA may convene an advisory committee to provide clinical insight on application review questions. Before approving a NDA or BLA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a NDA or BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After the FDA evaluates a NDABLA or BLANDA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be manufactured,produced and of select clinical trial sites, the FDA may issue an approval letter or a Complete Response letter.Letter (“CRL”). An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A Complete Response letterCRL will describe all of the deficiencies that the FDA has identified in the NDABLA or BLA,NDA, except that where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the Complete Response letterCRL without first conducting required inspections, testing submitted product lots, and/or reviewing proposed labeling. In issuing the Complete Response letter,CRL, the FDA may recommend actions that the applicant might take to place the NDABLA or BLANDA in condition for approval, including requests for additional information or clarification, including the potential requirement for additional clinical studies.clarification. The FDA may delay or refuse approval of a BLA or NDA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product for human therapeutic or prophylactic use is granted, such approval will be granted for particular indications and may entailinclude limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the NDA or BLA with a Risk Evaluation and Mitigation Strategy, or REMS, to ensure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a product and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more Phase 4 post-market studies andor post-market surveillance to further assess and monitor the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post-marketing studies.
Post-Approval Requirements
Any productsdrugs or biologics manufactured or distributed by us pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, reporting of adverse experiences, periodic reporting, product sampling and distribution, and advertising and promotion of the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, user fee requirements, under which the FDA assesses an annual program feefees for each product identified in an approved NDA or BLA. Manufacturersany marketed products. Biologic and drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs,cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMPscGMP and impose reporting requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMPscGMP and other aspects of regulatory compliance.
Post-market Enforcement
The FDA may withdraw approvalmarketing authorizations for drugs, biologics (including Section 361 HCT/Ps) and/or medical devices if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information;information, imposition of post-market studies or clinical studies to assess new safety risks;risks, or imposition of distribution restrictions or other restrictions under a Risk Evaluation and Mitigation Strategy (REMS) program.restrictions. Other potential consequences include, among other things:
•restrictions on the marketing or manufacturing of a product, complete withdrawal of the product from the market, or product recalls;
•recalls, fines, warning orletters, untitled letters, orclinical holds on post-approval clinical studies;
•studies, refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of existing product approvals;
•product seizureseizures or detention, or refusal of the FDA to permit the import or export of products;
•products, consent decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs;
•mandated modification of promotional materials and labeling andprograms, the issuance of corrective information;
•the issuance of safety alerts, Dear Healthcare Provider letters, press releases and other communications containing warnings or other safety information, about the product; or
•injunctions, or the imposition of civil or criminal penalties.
TheIn addition, the FDA closely regulates the marketing, labeling, advertising and promotion of drugs, biologics (including Section 361 HCT/Ps) and biologics.medical devices. A company can make only those claims relating to safety and efficacy, purity and potency that are cleared or approved by the FDA and in accordance with the provisions of the approvedauthorized label. However, companies may share truthful and not misleading information that is otherwise consistent with a product’s FDA approved labeling. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA, but physicians may not submit claims for reimbursement that are false or fraudulent. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use of their products.
International regulation of medical devices
Sales of medical devices outside the United States are subject to foreign government regulations, which vary substantially from country to country. In order to market our products in other countries, we must obtain regulatory approvals or certifications and comply with extensive safety and quality regulations in other countries. The time required to obtain approval or certification by a foreign country may be longer or shorter than that required for FDA approval and the requirements may differ significantly.
EU regulation of medical devices
The EUEuropean Union (“EU”) has adopted legislation,specific directives and regulations regulating the design, manufacture, clinical investigation, conformity assessment, labeling and adverse event reporting for medical devices.
Until May 25, 2021, medical devices were regulated by Council Directive 93/42/EEC (the “EU Medical Devices Directive”), which has been repealed and replaced by Regulation (EU) No 2017/745 (the “EU Medical Devices Regulation”). The majority of our current certificates have been granted under the EU Medical Devices Directive described below. However, as of May 26, 2021, some of the EU Medical Devices Regulation requirements apply in place of the formcorresponding requirements of directivesthe EU Medical Devices Directive with regard to be implemented in each Member State, concerning the regulationregistration of economic operators and of devices, post-market surveillance and vigilance requirements. Pursuing marketing of medical devices within the EU. The directives include, among others, the Medical Device Directive (Council Directive 93/42/EEC) that establishes certain requirements, such as the essential requirements, with which medical devices must comply before they can be commercialized in the European Economic Area (EEA) (which is comprisedEU will notably require that all of our devices not currently certified under the Member States ofnew requirements set forth in the EU plus Norway, Liechtenstein and Iceland). Medical Devices Regulation receive such certification when the current certificates expire.
Medical Devices Directive
Under the EU Medical DeviceDevices Directive, all medical devices are classified into four Classes,placed on the market in the EU must meet the relevant essential requirements in Annex I IIa, IIbto the EU Medical Devices Directive, including the requirement that a medical device must be designed and III, with Class I beingmanufactured in such a way that it will not compromise the lowest riskclinical condition or safety of patients, or the safety and Class III beinghealth of users and others. In addition, the highest risk. Underdevice must achieve the Medical Device Directive, a competent authority is nominatedperformance intended by the governmentmanufacturer and be designed, manufactured, and packaged in a suitable manner. The European Commission has adopted various standards applicable to medical devices. These include standards governing common requirements, such as sterilization and safety of each Member Statemedical electrical equipment and product standards for certain types of medical devices. There are also harmonized standards relating to monitordesign and ensuremanufacture. While not mandatory, compliance with these standards is viewed as the easiest way to satisfy the essential requirements as a practical matter as it creates a rebuttable presumption that the device satisfies that essential requirement.
To demonstrate compliance with the Directive. To demonstrate compliance of theiressential requirements in Annex I to the EU Medical Devices Directive, medical devices with the essential requirements,device manufacturers must undergo a conformity assessment evaluation,procedure, which varies according to the type of medical device and its (risk) classification. Generally, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. Except for certain types of low risklow-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment ofself-declare the conformity of its products with the essential requirements of the Medical Devices Directive,(except for any parts which relate to sterility or metrology), a conformity assessment evaluationprocedure requires the intervention of an organization accrediteda notified body. Notified bodies are independent organizations designated by a Member StateEU member states to assess the conformity of devices before being placed on the EEA to conduct conformity assessments, a so-called Notified Body. The Notified Bodymarket. A notified body would typically audit and examine a product’s technical dossiers and the manufacturers’ quality system (the notified body must presume that quality systems which implement the relevant harmonized standards – which is ISO 13485:2016 for Medical Devices Quality Management Systems – conform to these requirements). If satisfied that the manufacture, design and final inspectionrelevant product conforms to the relevant essential requirements, the notified body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE mark to the device, which allows the device to be placed on the market throughout the EU.
Throughout the term of the medical devices, along with conducting a technical reviewcertificate of data supportingconformity, the device’s safety and efficacy, before issuing a certification demonstratingmanufacturer will be subject to periodic surveillance audits to verify continued compliance with the essentialapplicable requirements. Both the quality system and the product are reviewed and certified. The Company is subject to annual surveillance auditsIn particular, there will be a new audit by the Notified Body and must undergo re-certification every 5 years. During these audits, (minor or major) non-conformitiesnotified body before it will renew the relevant certificate(s).
Medical Device Regulation
The regulatory landscape related to the essential requirement may be issued to the Company. The Company could potentially lose marketing authorization if these non-conformities are not remediated with the Notified Body. Significant modifications to the quality system or product changes for Class IIImedical devices must be submitted to the Notified Body for review prior to implementation. Non-significant changes are subject to review during the annual surveillance audits. Medical devices that comply with the essential requirements are entitled to bear the CE mark. Medical devices properly bearing the CE mark may be commercially distributed throughout the EEA. We have received CE certification from the British Standards Institute, a United Kingdom Notified Body, for conformity within the EU Medical Device Directive allowing uscontinues to place the CE mark on Durolane (Class III) and our Exogen bone healing system (Class IIa). Additional PMAs in individual EEA countries are sometimes required prior to marketing of a product. Failure to maintain the CE mark would preclude us from selling our products in the EEA, as could failure to comply with the specific requirements of the Member States.
evolve. On April 5, 2017, the European Parliament passed theEU Medical Devices Regulation which repealswas adopted with the aim of ensuring better protection of public health and replaces thepatient safety. The EU Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA Member States, the regulations would be directly applicable without the need for adoption of EEA Member State laws implementing them in all EEA Member States and are intended to eliminate current differences in the regulation of medical devices among EEA Member States. The Medical Devices Regulation among other things, is intended to establishestablishes a uniform, transparent, predictable and sustainable regulatory framework across the EEAEU for medical devices and in vitro diagnostic devices and ensure a high level of safety and health while supporting innovation.
The Unlike the EU Medical Devices Directive, the EU Medical Devices Regulation was meantis directly applicable in EU member states without the need for member states to become applicable three years after publication (in May 2020). However, on April 23, 2020, to takeimplement into national law. This aims at increasing harmonization across the pressure off EEA national authorities, notified bodies, manufacturers and other actors so they can focus fully on urgent priorities related to the COVID-19 pandemic, the European Council and Parliament adopted Regulation 2020/561, postponing the date of application of theEU.
The EU Medical Devices Regulation by one year (tobecame effective on May 2021). Once applicable, the26, 2021. The new regulations willRegulation among other things:
•strengthenstrengthens the rules on placing devices on the market (e.g., reclassification of certain devices and reinforcewider scope than the EU Medical Devices Directive) and reinforces surveillance once they are available;
•establishestablishes explicit provisions on manufacturers’ responsibilities for the follow-up onof the quality, performance and safety of devices placed on the market;
•improveestablishes explicit provisions on importers’ and distributors’ obligations and responsibilities;
•imposes an obligation to identify a responsible person who is ultimately responsible for all aspects of compliance with the requirements of the new regulation;
•improves the traceability of medical devices throughout the supply chain to the end-user or patient through the introduction of a unique identification number;number, to increase the ability of manufacturers and regulatory authorities to trace specific devices through the supply chain and to facilitate the prompt and efficient recall of medical devices that have been found to present a safety risk;
•setsets up a central database (Eudamed) to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and
•strengthenstrengthens rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional checka clinical evaluation consultation procedure by experts before they are placed on the market.
Devices lawfully placed on the market pursuant to the EU Medical Devices Directive prior to May 26, 2021 may generally continue to be made available on the market or put into service until their EU Medical Devices Directive certificate expires, provided that the requirements of the transitional provisions are fulfilled. In particular, the certificate in question must still be valid. However, even in this case, manufacturers must comply with a number of new or reinforced requirements set forth in the EU Medical Devices Regulation, in particular the obligations described below.
Regulation (EU) 2023/607 of the European Parliament and of the Council of 15 March 2023 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards the transitional provisions for certain medical devices and in vitro diagnostic medical devices. The Regulation introduces a staggered extension of the transition period provided for in Regulation (EU) 2017/745 on medical devices (MDR), subject to certain conditions. The transition period of medical devices to MDR has been extended until December 31, 2027 or December 31, 2028 depending on the classification of the medical device. The transition provisions covers products with CE certificates issued under the Medical Devices Directive (MDD) set to expire before the date of application. The amended Article 120(2), the extension of validity for these devices will, however, apply only if one of the following conditions is met:
(i)The manufacturer has signed a written agreement with a Notified Body for the conformity assessment of the device in question at the moment of expiry,
(ii)a national competent authority has granted a derogation from the applicable conformity assessment procedure in accordance with Article 59 of the MDR; or
(iii)a national competent authority has required the manufacturer to carry out the conformity assessment procedure within a specific time period in accordance with Article 97 of the MDR.
The EU Medical Devices Regulation requires that before placing a device, other than a custom-made device, on the market, manufacturers (as well as other economic operators such as authorized representatives and importers) must register by submitting identification information to the electronic system (“Eudamed”), unless they have already registered. The information to be submitted by manufacturers (and authorized representatives) also includes the name, address and contact details of the person or persons responsible for regulatory compliance. The new EU Medical Devices Regulation also requires that before placing a device, other than a custom-made device, on the market, manufacturers must assign a unique identifier to the device and provide it along with other core data to the unique device identifier (“UDI”) database. These new rulesrequirements aim at ensuring better identification and procedures may resulttraceability of the devices. Each device – and as applicable, each package – will have a UDI composed of two parts: a device identifier (“UDI-DI”) specific to a device, and a production identifier (“UDI-PI”) to identify the unit producing the device. Manufacturers are also notably responsible for entering the necessary data on Eudamed, which includes the UDI database, and for keeping it up to date. The obligations for registration in increased regulatory oversightEudamed will become applicable at a later date (as Eudamed is not yet fully functional). Until Eudamed is fully functional, the corresponding provisions of ourthe EU Medical Devices Directive continue to apply for the purpose of meeting the obligations laid down in the provisions regarding exchange of information, including, and in particular, information regarding registration of devices and economic operators.
All manufacturers placing medical devices into the market in the EU must comply with the EU medical device vigilance system. Under this may, in turn, increasesystem, serious incidents and Field Safety Corrective Actions (“FSCAs”) must be reported to the costs, time and requirementsrelevant authorities of the EU member states. Manufacturers are required to take FSCAs defined as any corrective action for technical or medical reasons to prevent or reduce a risk of a serious incident associated with the use of a medical device that we need to meet in order to maintain or place such devicesis made available on the EEA market. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device.
Further, theThe advertising and promotion of our products in the EEAmedical devices is subject to the laws of individual EEA Member States implementingsome general principles set forth in EU legislation. According to the EU Medical Devices Directive,Regulation, only devices that are CE marked may be marketed and advertised in the EU in accordance with their intended purpose. Directive 2006/114/EC concerning misleading and comparative advertising and Directive 2005/29/EC on unfair commercial practices, as well as other EEA Member Statewhile not specific to the advertising of medical devices, also apply to the advertising thereof and contain general rules, for example, requiring that advertisements are evidenced, balanced and not misleading. Specific requirements are defined at a national level. EU member states’ laws governingrelated to the advertising and promotion of medical devices. These lawsdevices, which vary between jurisdictions, may limit or restrict the advertising and promotion of our products to the general public and may impose limitations on our promotional activities with healthcare professionals.
Many EU member states have adopted specific anti-gift statutes that further limit commercial practices for medical devices, in particular vis-à-vis healthcare professionals and organizations. Additionally, there has been a recent trend of increased regulation of payments and transfers of value provided to healthcare professionals or entities and many EU member states have adopted national “Sunshine Acts” which impose reporting and transparency requirements (often on an annual basis), similar to the requirements in the United States, on medical devicesdevice manufacturers. Certain countries also mandate implementation of commercial compliance programs.
The aforementioned EU rules are generally applicable in the European Economic Area (“EEA”) which consists of the 27 EU member states plus Norway, Liechtenstein and Iceland. Legislation has been approved to extend the transition dates for the EU MDR to 2027/2028. Despite the extension, the notified bodies that are responsible for implementing the EU MDR guidelines must still adopt these changes into their current framework of procedures, which could take additional time.
Other countries
Many other countries have specific requirements for classification, registration and post marketing surveillance that are independent of the countries already listed. We obtain what we believe are the appropriate clearances for Durolane and our Exogen bone healing systemproducts and conduct our business in accordance with the applicable laws of each country. This landscape is constantly changing, and we could be found in violation if we interpret the laws incorrectly or fail to keep pace with changes. In the event of either of these occurrences, we could be instructed to recall products, cease distribution and/or be subject to civil or criminal penalties.
Anti-kickback false claimsStatute, False Claims Act and other healthcare laws
In addition to FDA restrictions on the marketing of pharmaceutical, biological and medical device products, weWe are also subject to a number of U.S. laws regulating healthcare regulationfraud, waste, and enforcement byabuse (“FWA”) including without limitation the federal governmentAnti-Kickback Statute and the statesfederal Physician Self-Referral Law (known as the Stark Law), the Civil False Claims Act, the civil prohibition on beneficiary inducements, and foreign governmentsthe Health Insurance Portability and authorities in the locations in which we conduct our business.Accountability Act of 1996 (“HIPAA”), as well as numerous state laws regulating healthcare and insurance. These other agencies include,laws are enforced by, without limitation, CMS, other divisions of the U.S. Department of Health and Human Services (HHS)(“HHS”), including without limitation the HHS Office of Inspector General (“OIG”), the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, as well as state and local governments. Such agencies enforce a variety of laws which include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, data privacy and security and physician payment transparencyAmong other things, these laws, and regulations.
The federal Anti-Kickback Statute prohibits, among other things, any personothers aimed at curbing FWA, generally: (1) prohibit the provision of anything of value in exchange for the referral of patients or entity from knowingly and willfully offering, paying, soliciting or receiving any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, leaseorder, or orderrecommendation of any good, facility, item or service reimbursable, in whole or in part,reimbursed by Medicare, Medicaid or othera federal healthcare programs. The term “remuneration” has been broadly interpretedprogram, (including Medicare and Medicaid); (2) require that claims for payment submitted to include anything of value, including cash, improper discounts and free or reduced price items and services. Among other things, the Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical, biotechnology and medical device manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not meet the requirements of a statutory or regulatory exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business,programs be truthful; and (3) require the Anti-Kickback Statute has been violated. In addition, a person or entity does not need to have actual knowledgemaintenance of the statute or specific intent to violate it in order to have committed a violation. Violations are subject to civil monetary penalties up to $100,000 for each violation, plus up to three times the remuneration involved,certain government licenses and may result in criminal fines of up to $100,000 and imprisonment of up to ten years, or exclusion from Medicare, Medicaid or other governmental programs. Further, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute also constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.permits.
Federal law also includes a provision commonly known as the “Stark Law,” which prohibits a physician from referring Medicare or Medicaid patients to an entity providing “designated health services,” which includes durable medical equipment, if the physician or immediate family member of the physician, has an ownership or investment interest or compensation arrangement with such entity that does not comply with the requirements of a Stark exception. Violation of the Stark Law could result in denial of payment, disgorgement of reimbursements received under a non-compliant arrangement, civil penalties, and exclusion from Medicare, Medicaid or other governmental programs.
The federal false claims and civil monetary penalties laws, including the civil False Claims Act, prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to or approval by the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. Actions under the False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the U.S. government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical, biotechnology and medical device companies throughout the country. Manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by for example, in connection with the promotion of products for unapproved or off-label uses and other sales and marketing practices. The government has obtained multi-million and multi-billion dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. When an entity is determined to have violated the federal civil False Claims Act, the government may impose civil fines and penalties ranging from $11,181 to $22,363 for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs. In addition, companies found liable under the False Claims Act have been forced to implement extensive corrective action plans, and have often become subject to consent decrees or corporate integrity agreements, severely restricting the manner in which they conduct their business and imposing ongoing reporting and disclosure obligations.
The federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Given the significant size of actual and potential settlements, it is expected that governmental authorities will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws. For drugs that are covered under Medicare Part B, the manufacturer must report average sales price (ASP), to CMS on a quarterly basis. Failure to report this information in a timely and accurate manner can lead to substantial civil and criminal penalties and to liability under the False Claims Act.
In July of 2018 we became aware of allegations that certain of our sales personnel may have been completing Section B of the Certificate for Medical Necessity form (CMN) required in connection with Medicare claims for the Exogen system, which, under federal law, must be completed by the physician and/or physician staff.
Together with our outside counsel, we initiated an investigation into these allegations, and we determined that the CMN forms for a portion of Medicare claims for the Exogen system were in fact improperly completed by our sales representatives, some of which also failed to meet CMS coverage requirements. As a result of our findings we made a self-disclosure on November 30, 2018 to the Office of Inspector General of the HHS (OIG), under the Provider Self-Disclosure Protocol. Our self-disclosure disclosed the extent of our findings relative to the inappropriate completion of CMN forms by our sales personnel and offered to make repayment for such claims which failed to meet CMS coverage requirements and which we submitted to the Medicare program between October 1, 2012 and September 30, 2018, the statutory period applicable to such conduct. The total value of impacted claims was $30.1 million in the aggregate.
In October 2019, our outside counsel received a letter from the Office of the United States Attorney in the Middle District of North Carolina (USAO) stating that the USAO would be working with the OIG to resolve our self-disclosure. After settlement discussions with the USAO and OIG, on January 25, 2021 we reached an agreement in principle with the USAO and the OIG with respect to the submission of Medicare claims that did not meet CMS coverage requirements and for which our sales representatives completed Section B of the CMN forms. On February 22, 2021, we finalized all terms related to the settlement and entered into a formal settlement agreement with the USAO and OIG consistent with our previous agreement in principle and which included releases from associated False Claims Act liability and further Civil Monetary Penalties that are customary in self-disclosures of this type. Under the agreement, we resolved the potential liability related to our self-disclosure for $3.6 million, of which $2.4 million had already been paid through our 2019 return of overpayments described previously, leaving a net payment to be made of $1.2 million. We made payment of the $1.2 million net settlement amount due under the agreement on February 23, 2021. The settlement amount noted above was recorded in the consolidated financial statements for the year ended December 31, 2020.
In 2019, separate from the self-disclosure described above, as a result of our internal auditing of Exogen Medicare claims, we made repayments to our Medicare Administrative Contractors (MACs) for overpayments identified during such auditing totaling $7.5 million for the period October 1, 2012 through December 31, 2018. This amount reflected certain Medicare claims for Exogen for which we lacked adequate documentation of medical necessity consistent with Medicare coverage requirements. Similarly, in July of 2020, we made repayments to the MACs of $1.5 million after completing our internal auditing of Exogen Medicare claims for the period beginning January 1, 2019 through December 31, 2019. We maintain a reserve for reimbursement claims related to our Exogen system that may have been processed for payment without adequate medical records support. Our reserve is estimated using an extrapolation of an error rate from a statistical sample, which represents our best estimate as of the date of the financial statements, but because of the uncertainty inherent in such estimates, the ultimate repayment amounts may be materially different.
See Part I, Item 1A. Risk Factors—Risks related to government regulation—We may be subject to enforcement action if we engage in improper claims submission practices and resulting audits or denials of our claims by government agencies could reduce our net sales or profits.
The federal Health Insurance Portability and Accountability Act of 1996, as amended by Health Information Technology for Economic and Clinical Health Act (HITECH), and the regulations that implement both laws, collectively known as HIPAA, created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payers, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of these statutes or specific intent to violate them in order to have committed a violation.
Also, manyMany states have similar fraud and abuseFWA statutes or regulations that may be broader in scope and may apply regardless of payer, in addition to items and services reimbursed under Medicaid and other state programs.
The federal Anti-Kickback Statute (“AKS”) prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Several courts have interpreted the AKS’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the AKS has been violated. The AKS includes statutory exceptions and regulatory safe harbors that protect certain arrangements that would otherwise be prohibited by the statute. Failure to meet the requirements of an applicable AKS exception or safe harbor, however, does not render an arrangement illegal. Rather, the government must evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances, including the parties’ intent and the arrangement’s potential for abuse, and may be subject to greater scrutiny by enforcement agencies. Criminal penalties and administrative sanctions for violating the AKS include civil penalties of up to $120,816 per violation plus three times the amount of the improper remuneration, criminal penalties up to $100,000 per violation, prison terms, and exclusion from participation in the Federal health care programs. Under the Civil Monetary Penalties statute, physicians who pay or accept kickbacks also face penalties of up to $50,000 per kickback plus three times the amount of the prohibited remuneration.
The Stark Law prohibits a physician who has a financial relationship, or who has an immediate family member who has a financial relationship, with entities providing designated health services (“DHS”) from referring Medicare and Medicaid patients to such entities for the furnishing of DHS, unless an exception applies. The Stark Law also prohibits the entity from billing for any such prohibited referral. Unlike the AKS, the Stark Law is a strict liability statute, meaning that the law has been violated if the financial arrangement does not meet an applicable exception regardless of any intent by the parties to induce or reward referrals or the reasons for the financial relationship and the referral. If the Stark Law applies and the elements of an applicable exception have not been met, then the government is prohibited from making payment for such services and any amount paid pursuant to a non-compliant financial relationship must be reimbursed to the government. In addition to reimbursing the government any associated overpayment, violations of the Stark Law can lead to: (1) civil penalties of nearly $29,000 per claim (in 2023, adjusted annually for inflation); (2) three times the amount of damages suffered by the government; and (3) potential exclusion from participation in federal healthcare programs.
The Federal False Claims Act (“FCA”) prohibits a person who offersfrom knowingly presenting, or transferscaused to be presented, a false or fraudulent request for payment from the federal government, or from making a false statement or using a false record to have a claim approved. A claim includes “any request or demand” for money or property presented to the United States government. Moreover, the government may assert that a claim including items and services resulting from a violation of the AKS or the Stark Law constitutes a false or fraudulent claim for purposes of the civil False Claims Act. In addition, knowingly concealing or decreasing an obligation to pay the government (or reimburse, in the case of an overpayment) can create liability under the reverse false claims provision of the statute. Overpayments must be repaid to the government within 60 days of the time the provider or supplier determines (or reasonably should have determined through the exercise of reasonable diligence) that it received an overpayment. Penalties for a violation of the FCA include fines for each false claim, plus up to three times the amount of damages caused by each false claim. Private individuals, known as qui tam relators, also have the ability to bring actions under these false claims laws in the name of the government alleging false and fraudulent claims presented to or paid by the government (or other violations of the statutes). Such realtors, often current or former employees or competitors, share in any amounts paid to the government in fines or settlement.
Further, the Civil Monetary Penalties Statute authorizes the imposition of civil monetary penalties, assessments and exclusion against an individual or entity based on a variety of prohibited conduct, including, but not limited to offering remuneration to a Medicare or Medicaidfederal healthcare program beneficiary any remuneration, including waivers of co-payments and deductible amounts (or any part thereof), that the personindividual or entity knows or should know is likely to influence the beneficiary’s selection ofbeneficiary to order or receive healthcare items or services from a particular provider practitioner or supplier of Medicare or Medicaid payable items or services may be liable for civil monetary penalties of up to $20,866 for each wrongful act.(also known as “beneficiary inducements”). Moreover, in certain cases, providers who routinely waive co-payments and deductibles for Medicare and Medicaid beneficiaries can also be held liable under the Anti-Kickback StatuteAKS and civil False Claims Act, which can impose additional penalties associated with the wrongful act.FCA. One of the statutory exceptions to the beneficiary inducements prohibition is non-routine, unadvertised waivers of co-payments or deductible amounts based on individualized determinations of financial need or exhaustion of reasonable collection efforts. The OIG emphasizes, however, that this exception should only be used occasionally to address special financial needs of a particular patient. Although this prohibition applies only to federal healthcare program beneficiaries, the routine waivers of co-payments and deductibles offered to patients covered by commercial payers may implicate applicable state laws related to, among other things, unlawful schemes to defraud, excessive fees for services, tortious interference with patient contracts and statutory or common law fraud. To the extent our patient assistance programs are found to be inconsistent with applicable laws, we may be required to restructure or discontinue such programs, or be subject to other significant penalties.
Additionally, there has been a recent trend of increasedThe Health Insurance Portability and Accountability Act (“HIPAA”) also established federal and state regulation of payments made to physicians and certain other healthcare providers. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education and Reconciliation Act, or collectively, the Affordable Care Act, imposed,criminal statutes that prohibit, among other things, new annual reporting requirements throughknowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payers, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the Physician Payments Sunshine Actdelivery of or payment for covered manufacturers for certain paymentshealthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Many states in which we operate have also adopted similar fraud and “transfersabuse laws to those described above. The scope of value” providedthese laws and the interpretations of them vary from state to physiciansstate and teaching hospitals,are enforced by state courts and beginning in 2022, physician assistants, nurse practitionersregulatory authorities, each with broad discretion. Some state fraud and abuse laws apply to items or services reimbursed by any payer, including patients, employers and commercial insurers, not just those reimbursed by a federally or state funded healthcare program.
Violation of any of these laws or any other practitioners, as well as ownership and investment interests held by such providers and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interestsgovernmental regulations that apply may result in civil monetary penalties of up to an aggregate of $176,495 per year and up to an aggregate of $1.177 million per year for “knowing failures.” Covered manufacturers must submit reports by the 90th day of each calendar year. In addition, certain states require implementation of compliance programs and compliance with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, impose restrictions on marketing practices, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities and/or require tracking and reporting of gifts, compensation and other remuneration or items of value provided to physicians and other healthcare professionals and entities.
These laws impact the kinds of financial arrangements we may have with hospitals, physicians or other potential purchasers of our products. They particularly impact how we structure our sales offerings, including discount practices, customer support, education and training programs, physician consulting, research grants and other arrangements. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws or regulations that apply to us, we may be subject tosignificant penalties, including, without limitation, potentially significantadministrative civil and criminal civil and/or administrative penalties, damages, disgorgement, fines, disgorgement,additional reporting requirements and compliance oversight obligations, in the event that a corporate integrity agreement or other agreement is required to resolve allegations of noncompliance with these laws, the curtailment or restructuring of operations, exclusion from participation in government healthcare programs imprisonment, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings and the curtailment and/or restructuringindividual imprisonment.
As a result of our sale or distribution of products in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post marketing requirements, including safety surveillance, anti-fraud and abuse laws and implementation of corporate compliance programs and reporting of payments or other transfers of value to healthcare professionals.
We also participate in state government-managed Medicaid programs as well as certain other qualifying federal and state government programs where discounts and mandatory rebates are provided to participating state and local government entities. In connection with several of these government programs, we are required to report prices to various government agencies. Pricing calculations vary among programs. The calculations are complex and are often subject to interpretation by the reporting entities, government agencies and the courts. Our methodologies for calculating these prices could be challenged under false claims laws or other laws. We could make a mistake in calculating reported prices and required discounts, which could result in retroactive liability to government agencies. Government agencies may also make changes in program interpretations, requirements or conditions of participation, some of which may have implications for amounts previously estimated or paid. If we make these mistakes or if governmental agencies make these changes, we could face, in addition to prosecution under federal and state false claims laws, substantial liability and civil monetary penalties, exclusionBy way of our products from reimbursement under government programs, criminal fines or imprisonment, or prosecutors may impose a Corporate Integrity Agreement, Deferred Prosecution Agreement, or similar arrangement.
Theexample, the Medicare Prescription Drug, Improvement and Modernization Act of 2003 (MMA)(“MMA”), requires that manufacturers report data to CMS on pricing of covered drugs reimbursed under Medicare Part B. These are generally drugs and biologicals, such as injectable products, that are administered “incident to” a physician service and in general are not self-administered. Effective January 1, 2005, ASP became the basis for reimbursement to physicians and suppliers for drugs and biologicals covered under Medicare Part B, replacing the average wholesale price (AWP)(“AWP”), provided and published by pricing services. In general, we must comply with all reporting requirements for any drug that is separately reimbursable under Medicare. TheOur SUPARTZ FX, product isGelsyn-3 and Durolane products are reimbursed under Medicare Part B and, as a result, we provide ASP data on this product to CMS on a quarterly basis.
Foreign Corrupt Practices Act
We are subject to the Foreign Corrupt Practices Act of 1977, as amended (“FCPA”). The FCPA prohibits U.S. companies and their representatives from processing, offering, or making payments of money or anything of value to foreign officials with the intent to obtain or retain business or seek a business advantage. In certain countries, the health care professionals we or our distributors regularly interact with may meet the definition of a foreign government official for the purposes of the FCPA. Our international activities create the risk of unauthorized payments or offers of payments by our employees, consultants and agents, including distributors, even though they may not always be subject to our control. Our existing safeguards may prove to be less than effective, and our employees, consultants, and agents may engage in conduct for which we might be held responsible. The FCPA also requires us to maintain accurate books and records and have a system of internal controls sufficient to, among other things, provide reasonable assurances that transactions are executed and assets are accessed and accounted for in accordance with management’s authorization. A determination that our operations or activities are not, or were not, in compliance with U.S. or foreign laws or regulations could result in the imposition of substantial fines, interruptions of business, loss of suppliers, vendor or other third-party relationships, termination of necessary licenses or permits, and legal or equitable sanctions. Other internal or governmental investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence. We are also subject to the UK and Brazilian equivalents, the UK Bribery Act and the Brazil Clean Company Act.
ISO Standards
We also operate and maintain a Quality Management System that is designed to comply with the requirements of International Standards ISO 13485: 2016 Medical Devices – Quality Management Systems. This system encompasses the principles of enhancing customer satisfaction through the effective application of processes for control, monitoring, and continual improvement, which is designed to ensure that we consistently meet or exceed customer expectations and applicable statutory/regulatory requirements.
Privacy and data protection laws in the United States
HIPAA and its implementing regulations, contain substantial restrictions and requirements with respect to the use and disclosure of certain protected health information (PHI)). These restrictions and requirementsWe are set forth in the HIPAA Privacy, Security and Breach Notification Rules.
In some of our operations, such those involving the acceptance of payments, we are a “covered entity” under HIPAA and therefore required to comply with the Privacy, Security and Breach Notification Rules and is subject to significant civila number of federal, state and criminal penalties for failure to do so. We also provide services to customersforeign laws and regulations that are covered entities themselvesgovern the collection, use, disclosure, and we are required to provide satisfactory written assurances to these customers through written “business associate” agreements that we will provide our services in accordance with HIPAA.
The Final Rule published by HHS Office for Civil Rights (OCR) in January 2013protection of health-related and effective March 23, 2013, modified the HIPAA Privacy, Security, Breach Notification and Enforcement Rules,other personal information, including revisions/additions made by the HITECH Act. The rule expanded thehealth information privacy and security requirements for business associateslaws, data breach notification laws, and consumer protection laws and regulations (e.g., Section 5 of the Federal Trade Commission Act). For example, HIPAA imposes obligations on “covered entities,” including certain healthcare providers, such as us, health plans, and healthcare clearinghouses, and their respective “business associates” that create, receive, maintain or transmit PHIindividually identifiable health information for or on behalf of a covered entities, increased penalties for noncomplianceentity, as well as their covered subcontractors with respect to safeguarding the privacy, security and strengthened requirements for reportingtransmission of breachesindividually identifiable health information. Entities that are found to be in violation of HIPAA, which may occur in connection with, among other things, a breach of unsecured PHI, among other changes. The rule also made business associates and their subcontractors directly liable for civil monetary penalties for impermissible uses and disclosures of PHI.
If we were to be found to have breached our obligations under HIPAA, we couldprotected health information (“PHI”), a complaint about privacy practices, or an audit by HHS, may be subject to enforcement actions by the OCRsignificant civil, criminal, and state health regulatorsadministrative fines, penalties, and lawsuits, including classdamages and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action law suits, by private plaintiffs. plan with HHS to settle allegations of HIPAA non-compliance.
In addition, OCR performs compliance audits in order to proactively enforcecertain state and foreign laws, including without limitation the HIPAACalifornia Consumer Privacy Act (“CCPA”), the California Privacy Rights Act (“CPRA”), General Data Protection Regulation (“GDPR”) and the United Kingdom General Data Protection Regulation (“UK GDPR”), govern the privacy and security standards. OCR has become an increasingly active regulator and has signaled its intention to continue this trend. OCR has the discretion to impose penalties without being required to attempt to resolve violations through informal means; further OCR may require companies to enter into resolution agreements and corrective action plansof personal information, including health-related information in certain circumstances, some of which impose ongoing compliance requirements. OCR enforcement activity can result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal resources. In addition to enforcement by OCR, state attorneys general are authorized to bring civil actions under either HIPAA or relevant state laws seeking either injunctions or damages in response to violations that threaten the privacy of state residents. Although we have implemented and maintained policies, processes and a compliance program infrastructure to assist us in complying with these laws and regulations and our contractual obligations, we cannot provide assurance regarding how these laws and regulations will be interpreted, enforced or applied to our operations.
In addition to HIPAA, we must adhere to state patient confidentiality laws that are not pre-empted by HIPAA, including those that are more stringent than HIPAA requirements. Numerousand many of which differ from each other state, federalin significant ways and foreign laws, including consumer protection laws and regulations, governmay not have the collection, dissemination, use, accesssame effect, thus complicating compliance efforts.
Failure to confidentiality and security of patient health information. In addition, Congress and some states are considering new laws and regulations that further protect the privacy and security of medical records or medical information. With the recent increase in publicity regarding data breaches resulting in improper dissemination of consumer information, all states have passed laws regulating the actions that a business must take if it experiences a data breach, such as prompt disclosure to affected customers. Generally,comply with these laws, are limited to electronic data and make some exemptions for smaller breaches. Congress has also been considering similar federal legislation relating to data breaches. The Federal Trade Commission (FTC) and states’ Attorneys General have also brought enforcement actions and prosecuted some data breach cases as unfair and/or deceptive acts or practices under the FTC Act. In addition to data breach notification laws, some states have enacted statutes and rules requiring businesses to reasonably protect certain types of personal information they hold or to otherwise comply with certain specified data security requirements for personal information. For example, the California Consumer Privacy Act (CCPA) took effect on January 1, 2020. The CCPA establishes a new privacy framework for covered businesses and provides new and enhanced data privacy rights to California residents, such as affording consumers the right to access and delete their information and to opt out of certain sharing and sales of personal information. The CCPA imposes severe statutory damages as well as a private right of action for certain data breaches thatwhere applicable, can result in the lossimposition of personal information. This private right of action is expected to increase the likelihood of, and risks associated with, data breach litigation. It remains unclear how various provisions of the CCPA will be interpreted and enforced. The CCPA contains an exemption for medical information governed by the California Confidentiality of Medical Information Act (CMIA) and for PHI collected by a covered entity significant civil and/or business associate governed by the privacy, security and breach notification rules established pursuant to HIPAA and HITECH, but the precise application and scope of this exemption is not yet clear, and the law may still apply to certain aspects of our business. Additionally, a new privacy law, the California Privacy Rights Act (CPRA), was approved by California voters in the November 3, 2020 election. The CPRA generally takes effect on January 1, 2023 and significantly modifies the CCPA, including by expanding consumers’ rights with respect to certain personal information, restricting use of sensitive personal information, which includes health information, and creating a new state agency to oversee implementation and enforcement efforts, potentially resulting in further uncertainty and requiring us to incur additional costs and expenses in an effort to comply. The CCPA and CPRA may lead other states to pass comparable legislation, with potentially greatercriminal penalties and more rigorous compliance requirements relevant to our business,private litigation. Privacy and that may not include exemptions for businesses subject to HIPAA. The effects of the CCPA,security laws, regulations, and other similar stateobligations are constantly evolving, may conflict with each other to complicate compliance efforts, and can result in investigations, proceedings, or federal laws, are potentially significant and may require us to modify our data processing practices and policies and to incur substantial costs and potential liability in an effort to comply with such legislation. As with HIPAA, any laws regulating the collection, dissemination, use, access to, confidentiality and security of personal information may apply directly to our business or indirectly by contract when we provide services to other companies. We intend to continue to comprehensively protect all consumer data and to comply with all applicable laws regarding the protection of this data.
Privacy and data protection laws in Europe
We are subject to European laws relating to our and our suppliers’, partners’ and subcontractors’ collection, control, processing and other use of personal data, such as data relating to an identifiable living individual, whetheractions that individual can be identified directly or indirectly. We and our suppliers, partners and subcontractors process personal data including in relation to our employees, employees of customers, trial patients, health care professions and employees of suppliers including health and medical information. The data privacy regime in the EU includes the General Data Protection Regulation (GDPR), the E-Privacy Directive (2002/58/EC) and national laws implementing or supplementing each of them.
The GDPR requires that personal data is only collected for specified, explicit and legal purposes as set out in the GDPR or local laws and the data may then only be processed in a manner consistent with those purposes. The personal data collected and processed must be adequate, relevant and not excessive in relation to the purposes for which it is collected and processed, it must be held securely, not transferred outside of the EEA unless certain steps are taken to ensure an adequate level of protection and must not be retained for longer than necessary for the purposes for which it was collected. In addition, the GDPR requires companies processing personal data to take certain organizational steps to ensure that they have adequate records, policies, security, training and governance frameworks in place to ensure the protection of data subject rights, including as required to respond to complaints and requests from data subjects. In addition, to the extent a company processes, controls or otherwise uses ‘special category’ personal data (including patients’ health or medical information, genetic information and biometric information), more stringent rules apply, further limiting the circumstances and the manner in which a company is legally permitted to process that data. Finally, GDPR provides a broad right for EU Member States to create supplemental national laws. Such laws and they are increasingly adopting different approaches to the role of the parties in clinical trials. Such laws, for example may relate to the processing of health, genetic and biometric data, which could further limit our ability to use and share such data or could cause our costs to increase and harm our business and financial condition.
From January 1, 2021, we are also subject to the GDPR and also the UK GDPR, which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, e.g. fines up to the greater of €20 million (£17.9 million) or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, and it is unclear how UK data protection laws and regulations will develop in the medium to longer term, and how data transfers to and from the United Kingdom will be regulated in the long term. Currently there is a four to six-month grace period agreed in the EU and UK Trade and Cooperation Agreement, ending 30 June 2021 at the latest, whilst the parties discuss an adequacy decision. However, it is not clear whether (and when) an adequacy decision may be granted by the European Commission enabling data transfers from EU member states to the United Kingdom long term without additional measures. These changes will lead to additional costssignificant civil and/or criminal penalties and increase our overall risk exposure.
In addition, the United Kingdom’s withdrawal from the European Union means that the United Kingdom will become a “third country” for the purposes of data transfers from the European Union to the United Kingdom following the expiration of the four to six-month personal data transfer grace period (from January 1, 2021) set out in the EU and UK Trade and Cooperation Agreement, unless a relevant adequacy decision is adopted in favor of the United Kingdom (which would allow data transfers without additional measures). These changes may require us to find alternative solutions for the compliant transfer of personal data into the United Kingdom.
We are also subject to evolving European lawsrestrictions on data export and electronic marketing. The rules on data export will apply when we transfer personal data to group companies or third parties outside of the EEA. For example, in 2015, the Court of Justice of the European Union (CJEU) ruled that the U.S.-EU Safe Harbor framework, one compliance method by which companies could transfer personal data regarding citizens of the EU to the United States, was invalid and could no longer be relied upon. European and U.S. negotiators agreed in February 2016 to a new framework, the EU-US Privacy Shield framework, which replaced the Safe Harbor framework, however, on July 16, 2020 the CJEU also invalidated the Privacy Shield framework as a method to transfer personal data from the EEA to US. While the CJEU upheld the adequacy of the standard contractual clauses (a standard form of contract approved by the European Commission as an adequate personal data transfer mechanism, and potential alternative to the Privacy Shield), it made clear that reliance on them alone may not necessarily be sufficient in all circumstances; this has created uncertainty. These changes will require us to review and amend the legal mechanisms by which we make and/ or receive personal data transfers to/ in the U.S. As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the standard contractual clauses cannot be used, and/or start taking enforcement action, we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer personal data between and among countries and regions in which we operate, it could affect the manner in which we provide our services, the geographical location or segregation of our relevant systems and operations, and could adversely affect our financial results.
We are also subject to evolving EU and UK privacy laws on cookies and e-marketing. In the European Union and the United Kingdom, regulators are increasingly focusing on compliance with requirements in the online behavioral advertising ecosystem, and current national laws that implement the ePrivacy Directive are highly likely to be replaced by an EU regulation known as the ePrivacy Regulation which will significantly increase fines for non-compliance. In the European Union and the United Kingdom, informed consent is required for the placement of a cookie or similar technologies on a user’s device and for direct electronic marketing. The GDPR also imposes conditions on obtaining valid consent, such as a prohibition on pre-checked consents and a requirement to ensure separate consents are sought for each type of cookie or similar technology. While the text of the ePrivacy Regulation is still under development, a recent European court decision and regulators’ recent guidance are driving increased attention to cookies and tracking technologies. If regulators start to enforce the strict approach in recent guidance, this could lead to substantial costs, require significant systems changes, limit the effectiveness of our marketing activities, divert the attention of our technology personnel, adversely affect our margins, increase costs and subject us to additional liabilities.
We are subject to the supervision of local data protection authorities in those jurisdictions where we are established or collecting data from EU residents. We depend on a number of third parties in relation to our provision of our services, a number of which process personal data on our behalf. With each such provider we enter into contractual arrangements to ensure that they only process personal data according to our instructions, and that they have sufficient technical and organizational security measures in place. Where we transfer personal data outside the EEA, we do so in compliance with the relevant data export requirements. As previously described, following the CJEU’s decision to invalidate Privacy Shield, we are now required to review and amend the legal mechanisms by which we make and/or receive personal data transfers to/in the U.S. As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the standard contractual clauses cannot be used, and/or start taking enforcement action, we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer personal data between and among countries and regions in which we operate, it could affect the manner in which we provide our services, the geographical location or segregation of our relevant systems and operations, and could adversely affect our financial results.
We take our data protection obligations seriously, as any improper, unlawful or accidental disclosure, loss, alteration or access to, personal data, particularly sensitive personal data, such as special category, could adversely affect our business and/or our reputation.
We may find it necessary or desirable to join self-regulatory bodies or other privacy-related organizations, particularly relating to biopharmacy and/or scientific research that require compliance with certain rules pertaining to privacy and data security.
There are costs and administrative burdens associated with compliance with GDPR and the resultant changes in the EU and EEA Member States’ national laws and the introduction of the e-Privacy Regulation once it takes effect. Any failure or perceived failure to comply with global privacy laws carries with it the risk of significant penalties and sanctions. These laws or new interpretations, enactments or supplementary forms of these laws, could create liability for us, could impose additional operational requirements on our business, and could affect the manner in which we use and transmit patient information and could increase our cost of doing business. Claims of violations of privacy rights or contractual breaches, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business.processing.
Coverage and reimbursement
Significant uncertainty existsOur products may be reimbursed by third-party payors, such as government programs, including Medicare and Medicaid, or private insurance plans and healthcare networks. As a result, demand for our products is and will continue to be dependent in part on the coverage and reimbursement statuspolicies of any pharmaceutical, biological or medical device product. Inthese payors. The manner in which reimbursement is sought and obtained varies based upon the United States and markets in other countries, patients who are prescribed treatments or undergo procedures for their conditionstype of payor involved and the providers performingsetting in which the services generally rely on third-party payers to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products unless coverageproduct is providedfurnished and reimbursement is adequate to cover a significant portion of the cost of our products or related procedures. Sales of any products will therefore depend, in part, on the availability of coverage and adequate reimbursementutilized. Reimbursement from third-party payers. Third-party payers include government authorities, managed care organizations, private health insurersMedicare, Medicaid and other organizations.
The process for determining whether a third-party payer will provide coverage for a product typically is separate from the process for setting the price of such product or for establishing the reimbursement rate that the payer will pay for the product once coverage is approved. Third-party payerspayors may limit coveragebe subject to specific products on an approved list, also known as a formulary, which might not include all of the FDA-approved products for a particular indication, or place products at certain formulary levels that result in lower reimbursement levels and higher cost-sharing obligation imposed on patients. A decision by a third-party payer not to cover any of our products or product candidates could reduce physician utilization of such products and adversely affect our business, results of operations and financial condition. Moreover, a third-party payer’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Additionally, coverage and reimbursement for products can differ significantly from payer to payer. One third-party payer’s decision to cover a particular medical product or service does not ensure that other payers will also provide coverage for the medical product or service, or will provide coverage at an adequate reimbursement rate. As a result, the coverage determination process will often require us to provide scientific and clinical support for the use of our products to each payer separately and can be a time-consuming process.
Third-party payers are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. Third-party payers may not consider our products to be medically necessary or cost-effective for certain indications or for all uses, andperiodic adjustments as a result of legislative, regulatory and policy changes, as well as budgetary pressures. Possible reductions in, or eliminations of, coverage or reimbursement by third-party payors, or denial of, or provision of uneconomical reimbursement for new products may not provide coverage foraffect our customers’ revenue and ability to purchase our products. In order to obtain and maintain coverage and reimbursement for our products and product candidates, we may need to conduct expensive clinical trials in order to demonstrate the medical necessity and cost-effectiveness of such products, in addition to the costs required to obtain regulatory approvals. If third-party payers do not consider a product to be cost-effective compared to other available therapies, they may not cover the product as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell our products at a profit. Any changes in coverage and reimbursement that further restrict coverage ofthe healthcare regulatory, payment or enforcement landscape relative to our products or lower reimbursement for procedures using our devices could materiallycustomers’ healthcare services have the potential to significantly affect our business. See Part I, Item 1A. Risk Factors—Risks relatedoperations and revenue. The Medicare program is expected to our business—If wecontinue to implement a new payment mechanism for certain durable medical equipment, prosthetics, orthotics, and supplies (“DMEPOS”) items via the implementation of its competitive bidding program. Bone growth therapy devices are unable to achieve and maintain adequate levels of coverage and/or reimbursement for our products, the procedures using our products, or any future products we may seek to commercialize, the commercial success of these products may be severely hindered.currently exempt from this competitive bidding process.
Outside of the United States, the pricing of medical devices and prescription pharmaceuticals is subject to governmental control in many countries. For example, in the EU, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular therapy to currently available therapies or so called health technology assessments, in order to obtain reimbursement or pricing approval. Other countries may allow companies to fix their own prices for products, but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Efforts to control prices and utilization of medical devices and pharmaceutical products could continue as countries attempt to manage healthcare expenditures, especially
Consolidated Appropriations Act
In July 2022, in light of the severe fiscal and debt crises experienced by many countries in the EU. There can be no assurance that any country that has price controls or reimbursement limitations for medical devices or pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products, if approved in those countries. See Part 1A. Risk Factors—Risks related to our business—Governments outside the United States may not provide coverage or reimbursement of our products, which may adversely affect our business, results of operations and financial condition.
Exogen reimbursement and order fulfillment
Our Exogen system is classified as durable medical equipment, meaning the product is used by the patient in the home and that the patient and/or insurance company, rather than the physician, is billed for the product. We bill third-party payers, such as private insurance or Medicare, on behalf of our patients and bill the patient for their co-payment obligations and deductibles. An internal team and external consultants assist with billing and processing orders for our Exogen system and has been trained in verifying case-by-case benefits, obtaining prior authorization and billing and collecting payments from payers. We also have a separate dedicated team of employees that provides customer support services for our Exogen system.
We have strong and established payer relationships, including the largest private payers in the United States. Based on our estimates, we are contracted as a provider with payers covering over 200 million lives. These contracts allow patients to access our Exogen system at a competitive rate and copay comparable to other suppliers and easing our administrative burden in processing at both authorization and when billing. Our Exogen system is reimbursed under Healthcare Common Procedure Coding System (HCPCS) code E0760.
Healthcare reform
Current and future legislative proposals to further reform healthcare or reduce healthcare costs may limit coverage for the procedures associatedconnection with the use of our products, or result in lower reimbursementConsolidated Appropriations Act, 2021 (“CAA”), the Centers for those procedures. The cost containment measures that payersMedicare and providers are instituting andMedicaid Services (“CMS”) began utilizing new pricing information the effect of any healthcare reform initiative implemented in the future could significantly reduce our revenues from the sale of our products. By way of example, the Affordable Care Act substantially changed healthcare financing and delivery by both governmental and private insurers and significantly impacted the pharmaceutical and medical device industries. The Affordable Care Act imposed, among other things, a new federal excise tax on the sale of certain medical devices, provided incentivesCompany reported to programs that increase the federal government’s comparative effectiveness research and implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models. Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act, including the permanent repeal of the federal excise tax on the sale of certain medical devices effective January 1, 2020. We expect there will be additional challenges and amendmentsit pursuant to the Affordable Care Act innewly adopted reporting obligations to adjust the future.
In addition, other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, included reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013. Subsequent legislative amendments related to the COVID-19 pandemic suspended this Medicare sequestration payment reduction from May 1, 2020 through March 31, 2021, but extended sequestration through 2030. On January 2, 2013, the American Taxpayer Relief Act of 2012, was signed into law, which, among other things, further reduced Medicare payments to several types of providers, including hospitals.
Moreover, payment methodologies may be subject to changes due to healthcare legislationproviders using our Durolane and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several U.S. Congressional inquiries and proposed and enacted federal legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare and review the relationship between pricing and manufacturer patient programs.
Individual states in the United States have also increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs.
In the EU, similar political, economic and regulatory developments have occurred or are being contemplated. In addition to continuing pressure on prices and cost containment measures, legislative developments at the EU or Member State level may result in significant additional requirements or obstacles that may increase operating costs. The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU Member States have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers.
We expect that additional foreign, state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure.Gelsyn-3 products.
Employee and Human Capital Resources
As of December 31, 2020,2023, we had approximately 700970 employees, none of whom were covered by collective bargaining agreements. Most of these employees are located in the United States with approximately 90120 located outside the United States. We believe that our relations with our employees are generally good.
We value our employees and regularly benchmark total rewards we provide, such as short and long term compensation, 401(k) contributions, health, welfare and quality of life benefits, paid time off and personal leave, against our industry peers to ensure we remain competitive and attractive to potential new hires. We seek to create a workplace environment that fosters personal and business successes by offering training and development programs, which further assist our current employees in meeting and exceeding our established standards of performance. performance, and a leadership development program specially designed to help our new leaders be successful in their expanded roles.
Additionally, throughto build on our culture of treating all individuals fairly and respectfully, we have established a Diversity, Equity and Inclusion Counsel,(“DE&I”) Council and formed several Employee Resource Groups (“ERGs”). The DE&I Council and ERGs are voluntary, employee-led groups of employees who come together in their workplace based on shared characteristics or life experiences. The stated mission of the DE&I Council is to foster a culture and identity that drives diversity, equity and inclusion as we engage and develop current employees and recruit future talent, all working together to build a transformative work environment. Our ERGs are generally intended to provide support, enhance career development, and contribute to personal development in the work environment. The goals of these and other similar initiatives is to encourage broad and diverse viewpoints to achieve the best outcomes for the patients, healthcare providers, and employees we serve.
Our Organizational Structure
Bioventus Inc. is a Delaware corporation formed on December 22, 2015 and functions as a holding company with no direct operations and our employees work directlyprincipal asset is the equity interest in BV LLC. We are headquartered in Durham, North Carolina. On February 16, 2021, we closed an initial public offering (“IPO”). Our IPO was conducted through what is commonly referred to as an umbrella partnership C corporation (“UP-C”) structure. In connection with the IPO and the UP-C structure, we completed a series of organizational transactions including, without limitation, the following:
•the limited liability company agreement of BV LLC was amended and restated (“Bioventus LLC Agreement”) to, among other things, (i) provide for a new single class of common membership interests in BV LLC (“LLC Interests”), (ii) exchange all of the then existing membership interests of the holders of BV LLC membership interests (“Original LLC Owners”) for LLC Interests and (iii) appoint Bioventus Inc. as the sole managing member of BV LLC; and
•the acquisition, by merger, of certain members of BV LLC (“Former LLC Owners”), for which we issued shares of Class A common stock as merger consideration (“Merger”).
•We amended and restated its certificate of incorporation to authorize Class A common stock, Class B common stock and undesignated preferred stock. Class B common stock has voting rights but no economic rights.
We have a majority economic interest, the sole voting interest in, and control the management of, BV LLC. As a result, we will consolidate the financial results of BV LLC and reports a non-controlling interest representing the LLC Interests held by Smith & Nephew, Inc. (“Continuing LLC Owner”). Refer to Part II, Item 8. Financial Statements and Supplementary Data—Notes to the Consolidated Financial Statements—Note 8. Stockholders’ equity of this Annual Report for additional information about the organizational transactions completed as part of the IPO.
Information about our Executive Officers
The following table sets forth information concerning our executive management teamofficers as of March 1, 2024:
| | | | | | | | | | | | | | |
| Name | | Age | | Position(s) |
| Robert E. Claypoole | | 52 | | President and Chief Executive Officer |
| Mark L. Singleton | | 55 | | Senior Vice President and Chief Financial Officer |
| Anthony D’Adamio | | 63 | | Senior Vice President and General Counsel |
| Katrina Church | | 62 | | Senior Vice President and Chief Compliance Officer |
Robert Claypoole joined Bioventus in January 2024 from Mölnlycke Health Care (“Mölnlycke”), a world-leading medical products and solutions company, where he served as Executive Vice President of Wound Care since July 2021. In this role, Mr. Claypoole had full responsibility for a $1.2 billion business. Before that, Mr. Claypoole served in several leadership positions with Mölnlycke from March 2017 to address any internal concernsJuly 2021, including Executive Vice President and continuously improvePresident, US for Mölnlycke and as an Officer of Mölnlycke Health Care US, LLC and Mölnlycke Manufacturing US, LLC. Prior to joining Mölnlycke in 2017, Mr. Claypoole served in various leadership roles at Medtronic Ltd. (now Medtronic plc (NYSE: MDT)), a global healthcare technology company, and Covidien, before it was acquired by Medtronic. Mr. Claypoole was Global Vice President & General Manager, Obesity & Metabolic Health (April 2016 to March 2017) and Global Vice President & General Manager of the waysSoft Tissue Repair & Hemostats business (December 2012 to April 2016). Before that, he was the Vice President, Executive Operations after serving as Vice President, Global Marketing while located in which weTrevoux, France. Prior to his time in France, Mr. Claypoole was the Vice President, US Marketing for the company’s Endomechanical & Intelligent Device business. Before joining Covidien in 2007, Mr. Claypoole held various marketing roles with increasing responsibility at Johnson & Johnson’s Vision Care division. Mr. Claypoole previously served on the board of directors of ZetrOz Inc. (January 2014 to January 2016) and the Association of periOperative Registered Nurses (December 2017 to December 2020). Mr. Claypoole received his Bachelor of Arts and his Masters of Business Administration from Cornell University. The Company’s Board of Directors (the “Board”) believes that Mr. Claypoole is well-qualified to serve on the Board considering the breadth of his experience across multiple global medical device markets and his extensive expertise in accelerating innovation, driving operational excellence, enhancing go-to-market strategies, and driving commercial execution and organizational effectiveness.
Mark Singleton has served as our employeesSenior Vice President and customers.Chief Financial Officer since March 2022. Mr. Singleton previously served as Vice President of Finance, Americas Strategic Business Units at Teleflex Inc. (“Teleflex”), a provider of specialty medical devices, from February 2021 to March 2022 and prior to that, served as Teleflex’s Vice President of Finance, Vascular Strategic Business Unit from 2014 to 2020. Prior to Teleflex, Mr. Singleton held multiple leadership roles at Lenovo Group Limited, a multinational technology company, including as Executive Director, Think Business Group Chief Financial Officer (2013-2014), Executive Director, Western Europe Chief Financial Officer (2011-2012), Executive Director, North America Chief Financial Officer (2007-2011) and Director, U.S. Finance Manager (2005-2007). Mr. Singleton received his Bachelor of Science from Purdue University and his Master of Business Administration from Duke University, Fuqua School of Business.
Anthony D’Adamio has served as our Senior Vice President and General Counsel since August 2017. Previously, Mr. D’Adamio was General Counsel and Secretary at Siemens Healthcare (now known as Siemens Healthineers AG) from January 2010 to August 2017 and served as Deputy General Counsel and Secretary of Siemens Healthcare Diagnostics from January 2007 to January 2010. Prior to that, Mr. D’Adamio was Senior Counsel within the Diagnostics Division of Bayer Healthcare LLC (now known as Siemens Healthineers Diagnostics) from January 2001 to December 2006. Mr. D’Adamio began his legal career at the law firm of Bond, Schoeneck & King before taking corporate legal positions with companies within the health insurance, pharmaceutical and biotechnology industries, including Group Health Incorporated, Quest Diagnostics and Covance Inc. Mr. D’Adamio holds a Juris Doctor from Howard University School of Law and a Bachelor of Arts from the State University of New York at Binghamton.
Katrina Church has served as our Chief Compliance Officer since August 2020. Prior to joining us, Ms. Church served in corporate counsel and compliance roles within the Merz Group of companies, most recently as Global Compliance Officer for Merz Pharma GmbH & Co KGaA, a privately-held pharmaceutical company, from March 2015 to August 2020. From June 1998 to December 2008, Ms. Church was Executive Vice President and General Counsel of Connetics Corporation, a specialty pharmaceutical company that was acquired by Stiefel Laboratories, Inc. in 2008. Ms. Church began her career as an attorney at Hopkins & Carley, a San Jose-based law firm. In 2020, Ms. Church was nominated for several industry awards for compliance training and received the 2020 Women in Compliance Award for “Most Impactful Compliance Training Programme of the Year” and the Brandon Hall 2020 Gold Medal for Excellence in Training. Ms. Church holds a Juris Doctor from New York University School of Law and a Bachelor of Arts in Comparative Literature from Duke University.
Available Information
Our filings with the Securities and Exchange Commission (“SEC”), including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, proxy statements for stockholder meetings, any registration statements, and amendments to those reports, are available free of charge on our website is located at www.bioventus.com.as soon as reasonably practicable after they are filed with, or furnished to, the SEC. Information on our website or connected to our website is not incorporated by referencesreference into this Annual Report. Our website is located at www.bioventus.com. Our SEC filings are also available on the SEC website at www.sec.gov.
Item 1A.Risk Factors.
Described below are certain risks that we believe apply to our business and the industry in which we operate. You should carefully consider each of the following risk factors in conjunction with other information provided in this Annual Report on Form 10-K (Annual Report)(“Annual Report”) and in our other public disclosures. The risks described below highlight potential events, trends or other circumstances that could adversely affect our business, financial condition, results of operations, cash flows, liquidity or access to sources of financing, and consequently, the market value of our Class A common stock. These risks could cause our future results to differ materially from historical results and from guidance we may provide regarding our expectations of future financial performance. The risks described below are those that we have identified as material and is not an exhaustive list of all the risks we face. There may be others that we have not identified or that we have deemed to be immaterial. All forward-looking statements made by us or on our behalf are qualified by the risks described below.
Risks related to our businessfinancial position
Our business mayIf we are unable to meet our current operating projections or secure other sources of liquidity, substantial doubt about our ability to continue to experience adverse impacts as a resultgoing concern may arise, which may negatively affect the market value of our Class A common stock.
The consolidated financial statements included elsewhere in this Annual Report have been prepared under the going concern basis of accounting, which presumes that the Company’s liquidation is not imminent. Based on our current operating plans and potential market and economic conditions, the Company believes it should be able to meet its obligations as they become due within the next twelve months from the date of issuance of the COVID-19 pandemic.
In 2020, the COVID-19 pandemic spread around the world andconsolidated financial statements included elsewhere in this Annual Report, as evaluated under generally accepted accounting principles in the U.S.United States.
While we were in compliance as of December 31, 2023 with the financial covenants as stated within the Credit and more recently, new variantsGuaranty Agreement dated as of December 6, 2019 (the “2019 Credit Agreement,” and, as amended by that certain Amendment No. 1 to Credit and Guaranty Agreement, dated as of August 29, 2021, by the Second Amendment to Credit and Guaranty Agreement, dated as of October 29, 2021, by the Third Amendment to Credit and Guaranty Agreement, dated as of July 11, 2022, by the Fourth Amendment to Credit and Guaranty Agreement, dated as of March 31, 2023 and by the Fifth Amendment, the “Amended 2019 Credit Agreement”), on January 18, 2024, the Company entered into Amendment No. 5 to the 2019 Credit Agreement (the “Fifth Amendment”) as part of its efforts to further improve its financial condition. The Fifth Amendment modified the financial covenant requirements contained in the 2019 Credit Agreement to provide additional financial covenant relief and included modifications to the Company’s leverage maintenance covenant and interest coverage ratio to provide financial and operational flexibility relative to its operating plan.
However, the Company has based the above assessment on assumptions of revenues, cash flows and operating costs that may prove to be wrong. In such event, the Company may need to seek further modifications to certain of the virus have emerged, somecovenants in the Amended 2019 Credit Agreement to avoid an event of which have showndefault or seek waivers of any potential noncompliance. Moreover, the Company has historically relied on its ability to be more contagious. The COVID-19 pandemic has had widespread, rapidly evolvingfund its operations primarily through cash flows from operations, as well as public and unpredictable impacts on global society, economies, financial markets and business practices. Federal and state governments have implemented measures in an effort to minimize the spread of the virus and ongoing effects of the pandemic, including social distancing, travel restrictions, border closures, limitations on public gatherings, mandatory closure or reduced capacity of business, work from home, supply chain logistical changes and other measures, which have caused global business disruptions and significant volatility in U.S. and internationalprivate debt and equity markets. financings, but there can be no assurances that such financing or funding will continue to be available to the Company on satisfactory terms, or at all. In addition, we cannot guarantee that financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities would dilute all of our stockholders. The incurrence of indebtedness would result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. The inability to secure alternative financing on acceptable terms or to obtain any additional required modifications to the Amended 2019 Credit Agreement in the event that we fail to meet our operating projections may raise substantial doubt about our ability to continue as a going concern and adversely affect our business and financial condition.
Our Amended 2019 Credit Agreement contains financial and operating restrictions that may limit our access to credit. If we fail to comply with its financial or other covenants, we may be required to repay the indebtedness, which may harm our liquidity.
We are subject to certain covenants under the Amended 2019 Credit Agreement, including, but not limited to:
•a minimum interest coverage ratio and a maximum debt leverage ratio requirement as defined in the Amended 2019 Credit Agreement;
•a minimum Liquidity (as defined in the Amended 2019 Credit Agreement) of not less than $10.0 million as of the end of each calendar month through October 29, 2025;
•restrictions on the declaration or payment of certain distributions on or in respect of our equity interests;
•restrictions on acquisitions, investments and certain other payments;
•limitations on the incurrence of new indebtedness;
•limitations on the incurrence of new liens on property or assets;
•limitations on transfers, sales and other dispositions;
•limitations on entering into transactions with affiliates; and
•limitations on making any material change in any of our business objectives that could reasonably be expected to have a material adverse effect on the repayment of our Amended 2019 Credit Agreement.
In the absence of a waiver from our lenders, any failure by us to comply with these covenants in the future might result in the declaration of an event of default, which could adversely affect our business, results of operations and financial conditionposition.
In addition, our indebtedness could have been, and may continuesignificant consequences on our financial position, including:
•requiring a substantial portion of our cash flows to be materially impacted duededicated to debt service payments instead of funding growth, working capital, capital expenditures, investments or other cash requirements;
•reducing our flexibility to adjust to changing business conditions or obtain additional financing;
•exposing us to the decrease in patient visitsrisk of increased interest rates as certain of our borrowings, including borrowings under our term loan, are at variable rates, making it more difficult for us to make payments on our indebtedness;
•restricting us from making strategic acquisitions or causing us to make non-strategic divestitures; and elective procedures
•limiting our ability to obtain additional financing for working capital, capital expenditures, debt service requirements and any future temporary cessations of elective procedures and could be further impacted by delays in payments from customers, supply chain interruptions, extended “shelter-in-place” orders or advisories, facility closuresgeneral corporate or other reasons related to the pandemic. Furthermore, the long-term impact of COVID-19 on our business will depend on many factors, including, but not limited to, the duration and severity of the pandemic, new and ongoing measures taken in response to the pandemic, the availability and effectiveness of vaccines to combat COVID-19, the impact on economic activity from the pandemic and actions taken in response and the resulting impact it has on our partners, patients and communities in which we operate, all of which continue to be uncertain. For example, there has been a decrease in patient visits to hospitals due to risk and fear of exposure to COVID-19, as well as decreases in, or temporary moratoriums on, elective procedures, which may be re-imposed in the future. In addition to lower sales, we have experienced decreased costs as a result of the pandemic including declines in travel and lower compensation related expenses. We have also implemented other various cost reduction initiatives and measures to safeguard liquidity, refer to purposes.
See Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Indebtedness for further detailsdiscussion concerning our indebtedness.
Failure to establish and maintain effective financial controls could cause us to have material weaknesses and financial misstatements due to error, which could adversely affect our business and stock price.
We are required to comply with the SEC’s rules implementing Sections 302, 404 and 906 of the Sarbanes-Oxley Act of 2002, which require management to certify financial and other information in our quarterly and annual reports, provide quarterly and annual management reports on the impacteffectiveness of COVID-19disclosure controls and procedures, and provide annual management reports on the effectiveness of internal controls over financial reporting. Though we are required to disclose changes made in our internal controls and procedures on a quarterly basis and assess internal controls over financial reporting on an annual basis, our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 until we are no longer an emerging growth company pursuant to the provisions of the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating.
We have undertaken various actions to comply with the requirements of being a public company. However, we cannot assure you that the measures we have taken to date, and actions we may take in the future, will be sufficient to prevent or avoid financial misstatements due to error or material weaknesses. If we identify any material weaknesses in the future that we cannot fully remediate, the accuracy and timing of our financial reporting may be adversely affected. Testing and maintaining financial controls can also divert our management’s attention from other matters that are important to the operation of our business. Ineffective disclosure controls and procedures or internal control over financial reporting could cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our Class A common stock.
Additionally, when evaluating our financial controls, we may identify material weaknesses in our internal controls that we may not be able to remediate in time to meet the applicable deadline imposed upon us for compliance with the requirements of Section 404. If we identify any material weaknesses in our internal controls over financial reporting or are unable to comply with the requirements of Section 404 in a timely manner, or if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal controls over financial reporting once we are no longer an emerging growth company, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our Class A common stock could be adversely affected, and we could become subject to investigations by the stock exchange on which our securities are listed, the SEC or other regulatory authorities, which could require additional financial and management resources.
We maintain our cash at financial institutions, often in balances that exceed federally insured limits.
We maintain the majority of our cash and cash equivalents in accounts at banking institutions in the United States that we believe are of high quality. Cash held in these accounts often exceed the Federal Deposit Insurance Corporation (“FDIC”) insurance limits. If such banking institutions were to fail, we could lose all or a portion of amounts held in excess of such insurance limitations. The FDIC took control of two such banking institutions, Silicon Valley Bank (“SVB”) on March 10, 2023 and Signature Bank (“Signature Bank”) on March 12, 2023. While we did have an account at SVB, we were able to recover all of our deposits when the FDIC stepped in and allowed us to transfer funds held at SVB to another bank without incurring any losses. In the event of failure of any of the financial institutions where we maintain our cash and cash equivalents, there can be no assurance that we would be able to access uninsured funds in a timely manner or at all. Any inability to access or delay in accessing these funds could adversely affect our business and financial position.
We might require additional capital to fund our financial and operating obligations and support business growth.
If our expected cash from operations together with available borrowings under our Amended 2019 Credit Agreement are not sufficient to fund our current financial and operating obligations, we might require additional capital. In addition, we intend to continue to make investments to support our business growth and might require additional funds to respond to business challenges or opportunities, including the need to further develop our current products and any new products, enhance our operating infrastructure, and acquire complementary businesses. Accordingly, we might need to engage in equity or additional debt financings to secure additional funds. If we raise additional funds through further issuances of equity or convertible debt securities, our existing stockholders could suffer significant dilution, and any new equity securities we issue could have rights, preferences and privileges superior to those of holders of our common stock. Any additional debt financing secured by us could involve restrictive covenants relating to our capital-raising activities and other financial and operational matters, which might make it more difficult for us to obtain additional capital and to pursue business opportunities, including potential acquisitions. In addition, we might not be able to obtain additional financing on terms favorable to us, if at all. If we are unable to obtain adequate financing or financing on terms satisfactory to us when we require it, our ability to continue to support our business growth and to respond to business challenges could be significantly limited.
Risks related to our business
We are currently subject to securities class action litigation and derivative shareholder lawsuits and may be subject to similar or other litigation in the future, which will require significant management time and attention, result in significant legal expenses and may result in unfavorable outcomes, which may have a material adverse effect on our business.business, operating results and financial condition, and negatively affect the price of our common stock.
ToWe are, and may in the extentfuture become, subject to various legal proceedings and claims that arise in or outside the COVID-19 disruptions continueordinary course of business. For example, on January 12, 2023, the Company and certain of its current and former directors and officers were named as defendants in a putative class action lawsuit filed in the Middle District of North Carolina, Ciarciello v. Bioventus, Inc., No. 1:23– CV – 00032-CCE-JEP (M.D.N.C. 2023). The complaint asserts violations of Sections 10(b) and 20(a) of the Exchange Act and of Sections 11 and 15 of the Securities Act and generally alleges that the Company failed to disclose certain information regarding rebate practices, its business and financial prospects, and the sufficiency of internal controls regarding financial reporting. The complaint seeks damages in an unspecified amount. On April 12, 2023, the Court appointed Wayne County Employees’ Retirement System as lead plaintiff. The plaintiff’s amended consolidated complaint was filed with the Court on June 12, 2023. On July 17, 2023, the defendants filed a motion to dismiss the complaint raising a number of legal and factual deficiencies with the amended consolidated complaint. In response to the defendants’ motion to dismiss, the lead plaintiff filed a second amended complaint on July 31, 2023. The defendants moved to dismiss the second amended complaint on August 21, 2023, which the Court granted in part and denied in part on November 6, 2023. The Court dismissed the plaintiff’s Securities Act claims, but allowed the plaintiff’s Exchange Act claims to proceed into discovery.
On October 4, 2023, certain of the Company’s current and former directors and officers were also named as defendants in a derivative shareholder lawsuit (in which the Company is a nominal defendant) filed in the United States District Court for the District of Delaware, Grogan, on behalf of Bioventus Inc., v. Reali, et.al., No. 1:23-CV-01099-RGA (D. Del. 2023). The complaint asserts violations of Section 14(a) of the Exchange Act, breaches of fiduciary duties and related state law claims, and a claim for contribution, and generally alleges the same purported misconduct as alleged in the Ciarciello case. On January 12, 2024, the Court agreed to stay the case pending resolution of the Ciarciello case.
On February 9, 2024, another plaintiff filed a derivative shareholder lawsuit against certain of the Company’s current and former directors and officers (in which the Company is a nominal defendant) filed in the United States District Court for the District of Delaware, Sanderson, on behalf of Bioventus Inc., v. Reali, et.al., No. 1:24-cv-00180-RGA (D. Del. 2024). Like the Grogan case, this case asserts violations of Section 10(b) of the Exchange Act, breaches of fiduciary duties and related state law claims, and a claim for contribution, and generally alleges the same purported misconduct as alleged in the Ciarciello case. The parties are in discussions to consolidate the two derivative matters and stay them on terms similar to those entered in the Grogan case.
The Company believes the claims alleged in each of the above matters lack merit and intends to defend itself vigorously. However, the outcome of these matters is not presently determinable, and any loss is neither probable nor reasonably estimable. The results of such lawsuits and any future legal proceedings cannot be predicted with certainty. Also, our assets may be insufficient to cover any claimed amounts that exceed our insurance coverage, and we may have to pay damage awards or otherwise may enter into settlement arrangements in connection with such claims. Any such payments or settlement arrangements in current or future litigation could have a material adverse effect on our business, operating results or financial condition. Even if the plaintiffs’ claims are not successful, current or future litigation could result in substantial costs and significantly and adversely impact our reputation and divert management’s attention and resources, which could have a material adverse effect on our business, operating results of operations and financial condition, it may also haveand negatively affect the effect of heightening many of the other risks described in Part I, Item 1A. Risk Factors, including risks relating to our ability to successfully commercialize new developed or acquired products or therapies, consolidation in the healthcare industry, disruptions in the supply or manufacturingprice of our products or their components, intensified pricing pressure as a resultcommon stock. In addition, such lawsuits may make it more difficult to finance our operations.
We are highly dependent on a limited number of products.
Our OA joint pain treatment and joint preservationHA products accounted for 53%43%, 54%42% and 49%51% of our total revenue for the years ended December 31, 2020, 20192023, 2022 and 2018,2021, respectively. We expect that sales of such products will continue to account for a substantial portion of our revenue, and therefore, our ability to execute our growth strategy and maintain profitability will depend upon the continued demand for these products. In addition, our supply and distribution agreements for Durolane, GELSYN-3 and SUPARTZ FX are subject to renewal and their terms end in December 2115, February 2026 and December 2028, respectively. If the supply and distribution agreements for any of our HA viscosupplementation therapiesproducts were terminated, our revenue would be impaired. If our OA joint pain treatment and joint preservationHA products fail to maintain their market acceptance for any reason, our business, results of operations and financial condition may be adversely affected.
Our long-term growth depends on our ability to develop, acquire and commercialize new products, line extensions or expanded indications.
Our industry is highly competitive and subject to rapid change and technological advancements. Therefore, it is important to our business that we continue to introduce new products and/or enhance our existing product offerings through line extensions or expanded indications. Developing, acquiring and commercializing products is expensive, time-consuming and could divert management’s attention away from our existing business. Even if we are successful in developing additional products, the success of any new product offering or enhancements to existing products will depend on several factors, including our ability to:
•properly identify and anticipate the needs of healthcare professionals and patients;
•develop and introduce new products, line extensions and expanded indications in a timely manner;
•distinguish our products from those of our competitors;
•avoid infringing upon the intellectual property rights of third-partiesthird parties and maintain necessary intellectual property licenses from third-parties;third parties;
•demonstrate, if required, the safety and efficacy of new products with data from preclinical studies and clinical trials;
•obtain clearance, approval, or approval,certification, if required, from the FDA and other regulatory agencies or notified bodies, for such new products, line extensions and expanded indications, and maintain full compliance with FDA and other regulatory requirements applicable to new devices or products or modifications of existing devices or products;
•provide adequate training to potential users of our products;
•market acceptance of our newly developed or acquired products or therapies;
•receive adequate coverage and reimbursement for our products; and
•maintain an effective and dedicated sales and marketing team.
If we are unsuccessful in developing, acquiring and commercializing new products or enhancing our existing product offerings through line extensions and expanded indications, our ability to increase our net saleslong-term growth may be impaired.negatively affected.
Additionally, our research and development efforts may require a substantial investment of time and resources before we are adequately able to determine the commercial viability of a new product, technology, material or other innovation. Such efforts may not result in the development of a viable product. In addition, even if we are able to successfully develop new active healing products, line extensions and expanded indications, these products may not produce sales in excess of the costs of development and they may be rendered obsolete by changing customer preferences or the introduction by our competitors of products embodying new technologies or features.
We may be unable to successfully commercialize newly developed or acquired products or therapies in the United States.
The commercial success of newly acquired or developed products, such as MOTYS, in the United States will depend upon the awareness and acceptance of such products among the medical community, including physicians and patients. Market acceptance will depend on a number of factors, including, among others:
•the perceived advantages and disadvantages of such products over existing therapies and other competitive treatments;
•availability of alternative treatments;
•inability to secure and maintain adequate coverage, including obtaining a unique reimbursement code;
•the extent to which physicians prescribe the Company’s products;
•the willingness of the target patient population to try new therapies;
•the strength of marketing and distribution support of the Company’s new products and competitive products;
•publicity concerning the Company’s new products, our existing products or competing products and treatments;
•pricing and cost effectiveness of such new therapies;
•the effectiveness of our sales and marketing strategies; and
•the willingness of patients to pay out-of-pocket in the absence of third-party reimbursement.
Our efforts to educate the medical community about the benefits of newly acquired or developed products may require significant resources and we may never be successful. If such newly acquired or developed products do not achieve an adequate level of acceptance by patients and physicians in the United States, our business, results of operations and financial condition may be adversely affected.
Demand for our existing portfolio of products and any new products, line extensions or expanded indications depends on the continued and future acceptance of our products by physicians, patients, third-party payers and others in the medical community.
We cannot be certain that our existing portfolio of products and any new products, line extensions or expanded indications that we develop will achieve or maintain market acceptance. With respect to our OA joint pain treatment and joint preservation products, third-partyThird-party payers may be reluctant to continue to cover our HA viscosupplementation therapiesproducts at their current prices. Further, new injectable therapies or oral medications may become available that help manage OA joint pain in a more convenient and/or cost effective manner than our HA viscosupplementation therapies. With respect to our BGS products,Surgical Solutions, new allograft, DBMs, synthetics, growth factors, or other enhancements to our existing implants may never achieve broad market acceptance, which can be affected by a lack of clinical acceptance of BGSsSurgical Solutions products and technologies, introduction of competitive treatment options which render BGSsSurgical Solutions products and technologies too expensive or obsolete and difficulty training surgeons in the use of BGSsSurgical Solutions products and technologies. Media reports or other negative publicity concerning both methods of tissue recovery from donors and actual or potential disease transmission from donated tissue may limit widespread acceptance by the medical community of our allografts, growth factor and DBMs, whether directed at these products generally or our products specifically. Unfavorable reports of improper or illegal tissue recovery practices by any participant in the industry, both in the United States and internationally, as well as incidents of improperly processed tissue leading to transmission of disease, may broadly affect the rate of future tissue donation and market acceptance of allograft based technologies by the medical community.
In addition, we believe that even if the medical community generally accepts our existing portfolio of products and any new products, line extensions or expanded indications, acceptance and recommendations by influential members of the medical community will be important to their broad commercial success. If the medical community does not broadly accept our products, we may not remain competitive in the market, which could adversely affect our business, results of operations and financial condition.
Our commercial success depends on our ability to differentiate the HA viscosupplementation therapies that we own or distribute from alternative therapies for the treatment of OA.
Our ability to achieve commercial success will, at least in part, depend on our ability to differentiate the HA viscosupplementation therapies that we own or distribute in such a way that physicians and patients will select them. The HA viscosupplementation therapies that we own or distribute could face competition from steroid injections, other HA viscosupplementation therapies, combination HA viscosupplementation/steroid therapies and alternate therapies for the treatment of OA, including those currently in development.
We expect that the HA viscosupplementation therapies that we own or distribute will continue to be used primarily after oral analgesics and steroid injections no longer provide adequate pain relief. In addition, the five and three injection HA viscosupplementation therapies that we distribute face competition from single injection therapies. We expect the three injection market to decline by a projected 3.1% CAGR and the five injection market to decline by a projected 13.6% CAGR from 2019 to 2024. Due to the convenience associated with the single injection treatments, it is expected that these products will continue to capture increasing market share of the HA viscosupplementation therapies market, which may adversely affect our business, results of operations and financial condition to the extent physicians and patients do not select Durolane, our single injection HA viscosupplementation therapy. There are also a number of combination HA viscosupplementation/steroid therapies currently in development. The American Association of Orthopedic Surgeons (AAOS), since the release of their May 2013 clinical practice guidelines, does not recommend the use of HA for patients with symptomatic knee OA. The evidence for the AAOS recommendation is based on two or more high quality studies with consistent findings for recommending for or against the intervention. The AAOS recommendation states that practitioners should follow a strong recommendation, such as this one, unless a clear and compelling rationale for an alternative approach is present. In May 2018, the Journal of the AAOS ranked the nonsteroidal anti-inflammatory drug naproxen the most effective in individual knee OA treatment for improving both pain and function. To the extent that any additional therapies receive approval or alternative therapies receive positive support from the AAOS or other physician associations, they could reduce the market share represented by HA viscosupplementation therapies for OA treatment and adversely affect our commercial success.
If we are unable to differentiate the HA viscosupplementation therapies we own or distribute from other therapies, physicians and patients may not be willing to use them or be willing to switch from existing therapies with which they are familiar. Once physicians incorporate a particular treatment into their practice, they may not alter their practice absent compelling clinical evidence of safety and/or effectiveness and/or significant pricing reimbursement advantages.
The proposed down-classification of non-invasive bone growth stimulators, including our Exogen, system, by the FDA could increase future competition for bone growth stimulators and otherwise adversely affect the Company’sour sales of Exogen.
On August 17,In 2020, the FDA published a Federal Register notice announcing its proposal to reclassify non-invasive bone growth stimulators, such as Exogen, from Class III medical devices to Class II with special controls. Class III devices are subject to the most stringent regulatory pathway for approval for medical devices requiring, among other things, rigorous clinical studies and pre-approval manufacturing review. Class II devices may be cleared for marketing by the FDA under the 510(k) pathway if they are determined to be substantially equivalent to a legally marketed predicate device. The 510(k) clearance process does not always require clinical testing, and is generally less onerous than the premarket approval process applicable to Class III devices. On September 8-9,Also in 2020, the Orthopaedic and Rehabilitation Devices Panel of the FDA Medical Devices Advisory Committee met and discussed FDA’s proposal. The Panel, whose authority is non-binding but nonetheless considered by FDA, ultimately voted in favor of the FDA’s proposal to down-classify non-invasive bone growth stimulators.
The FDA has proposed that any final order would become effective 30 days after publication. While the FDA has not yet finalized its proposal to down-classify non-invasive bone growth stimulators, should such down-classification occur now or in the future, we may face additional competition from new market entrants who would be able to pursue marketing authorization through the 510(k) clearance pathway instead of the more onerous and burdensome PMA approval process. Class II devices that qualify as durable medical equipment under the Medicare program may also be eligible for inclusion in Medicare’s competitive bidding program forfor durable medical equipment, prosthetics, orthoticsprosthetic and supplies.orthotic supplies (“DEMPOS”). As a result of down-classification, Exogen could face additional competition or we could receive lower reimbursement amounts, for Exogen, all of which could adversely affect our business, results of operations and financial condition.
If we are unable to achieve and maintain adequate levels of coverage and/or reimbursement for our products, the procedures using our products, or any future products we may seek to commercialize, the commercial success of these products may be severely hindered.
Our products are purchased by healthcare providers and customers who typically bill third-party payers such as government programs, including Medicare and Medicaid, or private insurance plans and healthcare networks, to cover all or a portion of the costs and fees associated with our products. Patients may also be billed for deductibles or co-payments in accordance with third-party payer policies. These third-party payers and insurers may deny reimbursement if they determine that a device or product provided to a patient or used in a procedure does not meet applicable payment criteria or if the policyholder’s healthcare insurance benefits are limited.
As required by law, the CMS, which administers the Medicare program, has continued efforts to implement a competitive bidding program for selected durable medical equipment, prosthetic and orthotic supplies items paid for by the Medicare program. In this program, Medicare rates are based on bid amounts for certain products in designated geographic areas, rather than the Medicare fee schedule amount. Bone growth stimulation products like our Exogen system are currently exempt from this competitive bidding process, but may be eligible for inclusion if the FDA’s proposed down-classification order becomes effective. We cannot predict which products from any of our businesses may ultimately be affected or whether or when the competitive bidding process may be extended to our businesses.
Limits Further, limits put on reimbursement by third-party payers, whether foreign or domestic, governmental or commercial, could make it more difficult to buy our products and substantially reduce, or possibly eliminate, patient access to our products. The healthcare industry in the United States has experienced a trend toward cost containment as government and private insurers seek to control rising healthcare costs by imposing lower payment rates and negotiating reduced contract rates with providers and suppliers.
There is no uniform policy ofPrivate payers may adopt coverage decisions and reimbursementpayment amounts determined by the Centers for our products or procedures using our products among third-party payersMedicare and Medicaid Services (“CMS”), the federal agency that administers the Medicare program in the United States, andas guidelines in setting their coverage and reimbursement for our productspolicies. In addition, CMS periodically reviews medical study literature to determine how the literature addresses certain procedures and procedures using our products can differ significantly from payer to payer. Further, these payers regularly review new and existing technologies for possible coverage and can, without notice, deny or reverse coverage for new or existing products and treatments. Third-party payers may not consider our products to be medically necessary or cost-effective for certain indications or off-label uses or for all uses, and as a result, may not provide coverage for the products. For example, Blue Cross Blue Shield Association’s Evidence Street platform issued a report in April 2018 questioning the efficacy of our Exogen system, which resulted in several non-coverage policies being issued by member organizations. Additionally, to the extent that third party payers decide that they are no longer willing to provide reimbursement for physician prescribed off-label uses of Exogen, sales may be negatively impacted. See Part I, Item 1A. Risk Factors—Risks related to government regulation—We may be subject to enforcement action if we engage in improper marketing or promotion of our products, and the misuse or off-label use of our products may harm our imagetherapies in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
We may also be required to conduct expensive clinical studies to justifyMedicare population. For some governmental programs, such as Medicaid, coverage and reimbursement and/or the level of reimbursement relativediffers from state to state. Medicaid payments to physicians, facilities and other therapies. In addition, should governmental authorities continue to enact legislation or adopt regulations that affect third-party coverage and reimbursement, access to our products and coverageproviders are often lower than payments by private or public insurers may be reduced. If third-party payers or insurers that currently cover or reimburse our products or the procedures in which they are used limit their coverage or reimbursement in the future, or if other third-party payers or insurers issue similar policies, this could impact our ability to selland some state Medicaid programs may not pay an adequate amount for the procedures performed with our products, force us to lower the price we charge for our products, and adversely affect our business, results of operations and financial condition.
Our ability to market and sell our products could be harmed by future actions byif any payment is made at all. If CMS, other government agencies or private payers lower their reimbursement rates or establish additional limitations on coverage of our products, or if any of the proposed drug pricing executive orders or legislative reforms are enacted, the commercial success of our products may be adversely affected.
Further, legislative or other regulatory reforms that have been adopted or may be adopted in the future may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies or other downward pressure on the pricing or reimbursement we or our customers receive for our products. For example, the Consolidated Appropriations Act, 2021 (“CAA”) was signed into law on December 27, 2020, and pursuant to diminish paymentsimplementing regulations promulgated by CMS, expanded price reporting obligations for manufacturers of certain products reimbursed under Medicare Part B beginning January 1, 2022, including all of our HA viscosupplements. In July 2022, CMS began utilizing the new pricing information we reported to it pursuant to these newly adopted reporting obligations to adjust the Medicare payment to healthcare providers using our Durolane and suppliers. For example,Gelsyn-3 products. As a result, the rates currently available for those products have been reduced from those previously available and will be subject to future reporting and adjustment, which may affect the demand for those products or our ability to sell them profitably. We cannot predict the extent to which this law, or other reimbursement reform proposals or other healthcare cost containment measures that might be enacted in the future, may impact the demand or commercial success of our HA viscosupplements and other products we sell or plan to commercialize in the future.
In addition, due to the manner in which rebates are calculated and paid under certain of our contracts with private payers, changes in the ASP for our HA viscosupplements may result in larger than expected rebates payments for the sale of these products. In addition, we are dependent on these payers to provide timely and accurate invoices for the rebates that we are obligated to pay under these contractual relationships. If the information is not received timely or is inaccurate, we may not be able to correctly forecast the amounts due under those agreements, which may adversely affect our operating results and financial condition.
CMS, in its ongoing implementation ofwhich administers the Medicare program, has continued efforts to implement a competitive bidding program for selected DEMPOS items paid for by the Medicare program. In this program, Medicare rates are based on bid amounts for certain products in designated geographic areas, rather than the Medicare fee schedule amount. Bone growth stimulation products like Exogen are currently exempt from this competitive bidding process, but may be eligible for inclusion if the FDA’s proposed down-classification order becomes effective. We cannot predict which products from any of our businesses may ultimately be affected or whether or when the competitive bidding process may be extended to our businesses.
CMS periodically reviews medical study literature to determine how the literature addresses certain procedures and therapies in the Medicare population. The impact that these assessments could have on Medicare or third-party payer coverage determinations for our products is currently unknown, but we cannot provide assurances that the resulting actions will not restrict Medicare or other insurance coverage for our products. In addition, there can be no assurance that we or our distributors will not experience significant coverage or reimbursement impediments in the future related to these or other programs and policies of CMS. Specifically, drug pricing reform legislation and executive orders, which could negatively affect the reimbursement rates paid for our HA viscosupplements, have been issued by the White House and proposed and enacted by Congress. For example, the Consolidated Appropriations Act, 2021(CAA), was signed into law on December 27, 2020, and will expand price reporting obligations for manufacturers of certain products reimbursed under Medicare Part B beginning, January 1, 2022. CMS could utilize the new pricing information to adjust Medicare payment for these products, which may include HA viscosupplements. We cannot predict how this law will be implemented by CMS, or the extent to which this law, or other proposals that may be enacted in the future, may impact the Medicare payment available for our HA viscosupplements.
Private payers may adopt coverage decisions and payment amounts determined by CMS as guidelines in setting their coverage and reimbursement policies. In addition, for some governmental programs, such as Medicaid, coverage and reimbursement differs from state to state. Medicaid payments to physicians, facilities and other providers are often lower than payments by other third-party payers and some state Medicaid programs may not pay an adequate amount for the procedures performed with our products, if any payment is made at all. If CMS, other government agencies or private payers lower their reimbursement rates, or if any of the proposed drug pricing executive orders or legislative reforms are enacted, the commercial success of our products may be adversely affected.
Our business may be adversely affected if consolidation in the healthcare industry leads to demand for price concessions or if a GPO,Group Purchasing Organization (“GPO”), third-party payers or other similar entities exclude us from being a supplier.
Healthcare costs have risen significantly over the past decade, which has resulted in or led to numerous cost reform initiatives by legislators, regulators and third-party payers. Cost reform has triggered a consolidation trend in the healthcare industry to aggregate purchasing power, which may increase requests for pricing concessions or risk vendor exclusion. For example, non-clinical staff at hospitals are increasingly involved in the evaluation of products and product purchasing decisions. In order for us to sell our products, we must convince such staff as well as physicians and hospitals that our products are attractive alternatives to competing products for use in surgical procedures. Additionally, GPOs, independentintegrated delivery networks (“IDNs”) and large single accounts may continue to use their market power to consolidate purchasing decisions for physicians. Third-party payers may also continue to use their market power to reduce the reimbursement for our products by increasing the rebates we are required to pay them when our products are covered, which may negatively impact our results. We expect that market demand, government regulation, third-party coverage and reimbursement policies and societal pressures will continue to change the healthcare industry worldwide, resulting in further business consolidations and alliances among our customers, which may exert further downward pressure on the prices of our products.
If we choose to acquire or invest in new businesses, products or technologies, we
We may be unable to complete theseproposed acquisitions or to successfully integrate themproposed or recent acquisitions in a cost-effective and non-disruptive manner.
Our success depends on our ability to enhance and broaden our product offerings in response to changing customer demands, competitive pressures and advances in technologies. We continue to search for viable acquisition candidates or strategic alliances that would expand our market sector and/or global presence, as well as additional products appropriate for current distribution channels. Accordingly, we have previously and may in the future pursue the acquisition of, or joint ventures relating to, new businesses, products or technologies instead of developing them internally. For example, we entered into an OptionOur future success will depend, in part, upon our ability to manage the expanded business following these acquisitions, including challenges related to the management and Equity PurchaseAgreementmonitoring of new operations and associated increased costs and complexity associated with CartiHeal providing for, among other things, an exclusive option to acquire the company under certain terms and conditions as described above. See “Business—Development and Clinical Pipeline—Treatment of Cartilage for Osteochondral defects—CartiHeal (developer of Agili-C) investment and option and equity purchase agreement.” We are also exploring a possible acquisition of a medical device company, referred to herein as the Target. We were recently notified that a minority shareholder of the Target has filed a complaint with the Court of Chancery of the State of Delaware contesting the Potential Transaction, as well as related motions to expedite proceedingsMisonix, Bioness, and issue a temporary restraining order. We understand that the Target intends to defend the action vigorously, but there can be no assurance that this action will not impact the timing, terms or likelihood of closing of the Potential Transaction.other potential acquisitions. Other risks involving potential future and completed acquisitions and strategic investments include:
•risks associated with conducting due diligence;
•problems integrating the purchased technologies, products or business operations;
•inability to achieve the anticipated synergies and overpaying for acquisitions or unanticipated costs associated with acquisitions;
•invalid net sales assumptions for potential acquisitions;
•issues maintaining uniform standards, procedures, controls and policies;
•diversion of management’s attention from our core business;
•adverse effects on existing business relationships with suppliers, distributors and customers;
•risks associated with entering new markets in which we have limited or no experience;
•potential loss of key employees of acquired businesses; and
•increased legal, accounting and compliance costs.
We compete with other companies for these opportunities, and we may be unable to consummate such acquisitions or joint ventures on commercially reasonable terms, or at all. In addition, acquired businesses may have ongoing or potential liabilities, legal claims (including tort and/or personal injury claims) or adverse operating issues that we fail to discover through due diligence prior to the acquisition. Even if we are aware of such liabilities, claims or issues, we may not be able to accurately estimate the magnitude of the related liabilities and damages. In particular, to the extent that prior owners of any acquired businesses or properties failed to comply with or otherwise violated applicable laws or regulations, failed to fulfill their contractual obligations to their customers, or failed to satisfy legal obligations to employees or third parties, we, as the successor, may be financially responsible for these violations and failures and may suffer reputational harm or otherwise be adversely affected. Acquisitions also frequently result in the recording of goodwill and other intangible assets which are subject to potential impairment in the future that could harm our financial results. If we were to issue additional equity in connection with such acquisitions, this may dilute our stockholders.
Pricing pressure from our competitors or hospitals may affect our ability to sell our products at prices necessary to support our current business strategies.
Medical device companies, healthcare systems, IDNs and GPOs have intensified competitive pricing pressure as a result of industry trends and new technologies. Purchasing decisions are gradually shifting to hospitals, IDNs and other hospital groups, with surgeons and other physicians increasingly acting only as “employees.” Changes in the purchasing behavior of hospitals or the amount that third-party payers are willing to reimburse our customers for procedures using our products, including those as a result of healthcare reform initiatives, could create additional pricing pressure on us. In addition to these competitive forces, we continue to see pricing pressure as hospitals introduce new pricing structures into their contracts and agreements, including fixed price formulas, capitated pricing and episodic or bundled payments intended to contain healthcare costs. If such trends continue to drive down the prices we are able to charge for our products, our profit margins will shrink, adversely affecting our business, results of operations and financial condition.
If we fail to successfully enter into purchasing contracts for our BGSSurgical Solutions products or engage in contract bidding processes internationally, we may not be able to receive access to certain hospital facilities and our sales may decrease.
In the United States, the hospital facilities where physicians treat patients with our BGSSurgical Solution products typically require us to enter into purchasing contracts. The process of securing a satisfactory contract can be lengthy and time-consuming and require extensive negotiations and management time. In certain international jurisdictions, from time to time, certain institutions require us to engage in a contract bidding process in the event that such institutions are considering making purchase commitments that exceed specified cost thresholds, which vary by jurisdiction. These processes are only open at certain periods of time, and we may not be successful in the bidding process. If we do not receive access to hospital facilities through these contracting processes or otherwise, or if we are unable to secure contracts or tender successful bids, our sales may stagnate or decrease and our operating results may be harmed. Furthermore, we may expend significant effort in these time-consuming processes and still may not obtain a purchase contract from such hospitals.
Governments outside the United States may not provide coverage or reimbursement of our products, which may adversely affect our business, results of operations and financial condition.
Acceptance of our products in international markets may depend, in part, upon the availability of coverage and reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country, and include both government-sponsored healthcare and private insurance. Our products may not obtain international coverage and reimbursement approvals in a timely manner, if at all, which may require consumers desiring our products to purchase them directly. Third-party coverage and reimbursement for our products or any of our products in development for which we may receive regulatory approval may not be available or adequate in international markets, which could adversely affect our business, results of operations and financial condition.
Our future growth depends on physician awareness of the distinctive characteristics, benefits, safety, clinical efficacy and cost-effectiveness of our products.
We focus our sales, marketing and training efforts on physicians, surgeons and other health care professionals. The acceptance of our products depends in part on our ability to educate physicians as to the distinctive characteristics, benefits, safety, clinical efficacy and cost-effectiveness of our products compared to alternative products, procedures and therapies. If physicians, surgeons or other healthcare professionals are not properly trained, they may misuse or ineffectively use our products, which may result in unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us. In addition, a failure to educate physicians, surgeons or other healthcare professionals regarding our products may impair our ability to achieve market acceptance of our products.
We compete and may compete in the future against other companies, some of which have longer operating histories, more established products or greater resources than we do, which may prevent us from achieving increased market penetration or improved operating results.
The medical device industry is characterized by intense competition, subject to rapid change and is significantly affected by market activities of industry participants, new product introductions and other technological advancements. We believe that our competitors have historically dedicated and will continue to dedicate significant resources to promote their products or to develop new products. We have competitors in the United States and internationally, including major medical device and pharmaceutical companies, biotechnology companies and universities and other research institutions.
These companies and other industry participants may develop alternative treatments, products or procedures that compete directly or indirectly with our products. If alternative treatments are, or are perceived to be, superior to our products, sales of our products could be adversely affected and our results of operations could suffer. Our competitors may also develop and patent processes or products earlier than we can or obtain regulatory clearanceclearances, approvals or approvalscertifications for competing products more rapidly than we can, which could impair our ability to develop and commercialize similar processes or products.
Many of our current and potential competitors are major medical device and pharmaceutical companies that have substantially greater financial, technical and marketing resources than we do, and they may succeed in developing products that would render our products obsolete or noncompetitive. It is also possible that our competition will be able to leverage their large market share to set prices at a level below that which is profitable for us.
Some of our competitors enjoyhave several competitive advantages over us, including:
•greater financial, human and other resources for product research and development, sales and marketing and litigation;
•significantly greater name recognition;
•control of intellectual property and more expansive portfolios of intellectual property rights, which could impact future products under development;
•greater experience in obtaining and maintaining regulatory clearances, approvals or approvalscertifications for products and product enhancements;
•established relationships with hospitals and other healthcare providers, physicians, suppliers, customers and third-party payers;
•additional lines of products, and the ability to bundle products to offer greater incentives to gain a competitive advantage; and
•more established sales, marketing and worldwide distribution networks.
The potential introduction by competitors of products that compete with our existing or planned products may also make it difficult to market or sell our products. In addition, the entry of multiple new products and competitors may lead some of our competitors to employ pricing strategies that could adversely affect the pricing of our products and pricing in the market generally.
As a result, our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner, receive adequate coverage and reimbursement from third-party payers, and are safer, less invasive and more effective than alternatives available for similar purposes. If we are unable to do so, our sales or margins could decrease, which would adversely affect our business, results of operations and financial condition.
The reclassification of our HA products from medical devices to drugs in the United States by the FDA could negatively impact our ability to market these products and may require that we conduct costly additional clinical studies to support current or future indications for use of those products.
On December 18, 2018, the FDA published notice in the Federal Register announcing its intention to reconsider the appropriate classification of HA intra-articular products intended for the treatment of pain in OA of the knee. Although HA products intended for this use have previously been regulated as medical devices, in its notice the FDA stated that current published scientific literature supports that HA products achieve their primary intended purpose of treatment of pain in OA of the knee through biological action in the body which would require such products being classified as drugs. The FDA has encouraged organizations intending to submit applications for changes in indications for use, formulation, or route of administration of their HA products to obtain from the FDA an informal or formal classification and jurisdiction determination as a drug or device through a pre-request for designation or request for designation, respectively, prior to submission of such application. However, the FDA to date has taken no action to reclassify HA products from medical devices to drugs, or indicated what the potential ramifications would be for currently marketed HA products if a reclassification were to occur.
We currently market three HA products: Durolane, GELSYN-3 and SUPARTZ FX. If the reclassification of HA products were to occur, the FDA may not allow us to continue to market theseour HA products without submitting additional clinical trial data, obtaining approval of a NDA for these products, or without otherwise complying with new conditions or limitations on how those products are marketed. Clinical testing can take years to complete, can be expensive and carries uncertain outcomes, and there is no guarantee that would be able to successfully obtain and maintain any required regulatory approvals. These new regulatory obligations could result in increased regulation of Durolane, GELSYN-3 and SUPARTZ FX and would subject theseour HA products to a new set of regulatory requirements to which they have not been previously subject. These changes could ultimately increase our costs, change levels of coverage and/or reimbursement for our HA products and adversely impact our business, results of operations and financial condition if they were to be implemented. See Part I, Item 1A. Risk Factors—Risks related to our business—business—If we are unable to achieve and maintain adequate levels of coverage and/or reimbursement for our products, the procedures using our products, or any future products we may seek to commercialize, the commercial success of these products may be severely hindered.”hindered.
Our ability to maintain our competitive position depends on our ability to attract, retain and motivate our senior management team and highly qualified personnel, and our failure to do so could adversely affect our business, results of operations and financial condition.
We believe that our continued success depends to a significant extent upon the skill, experience and performance of members of our senior management team, who have been critical to the management of our operations and implementation of our strategy, as well as our ability to continue to attract, retain and motivate additional executive officers, and other key employees and consultants, such as those individuals who are engaged in our research and development efforts. The replacement of any of our key personnel likely would involve significant time and costs and may significantly delay or prevent the achievement of our business objectives and could therefore adversely affect our business, results of operations and financial condition. In addition, we do not carry any “key person” insurance policies that could offset potential loss of service under applicable circumstances.
Competition for experienced employees in the medical device industry can be intense. To attract, retain and motivate qualified employees, we may utilize equity-based incentive awards such as employee stock options. If the value of such equity incentive awards does not appreciate as measured by the performance of the price of our Class A common stock, and ceasesas seen in current market conditions, such awards may cease to be viewed as a valuable benefit,and our ability to attract, retain and motivate our employees could be adversely impacted, which could adversely affect our business, results of operations and financial condition and/or require us to increase the amount we expend on cash and other forms of compensation.
Since inception, our history of operations has included periods of net losses, and we may not be able to sustain profitability.
For the years ended December 31, 2020, 2019 and 2018, we had net income from continuing operations of $14.7 million, $8.1 million and $4.4 million, respectively. We had an accumulated deficit of $144.5 million and $141.7 million as of December 31, 2020 and 2019, respectively. Our ability to generate sufficient net sales from our existing products or from any of our products indevelopment or products that we acquire, in order to sustain profitability, is uncertain, and, since inception, our history of operations has previously included periods of net loss. We expect that our operating expenses will continue to increase as we continue to develop, enhanceand commercialize new products and incur additional operational costs associated with being a public company. Furthermore, we may not be able tosustain or increase profitability on an ongoing basis. If we do not achieve sustained profitability, it will be more difficultfor us to finance our business and accomplish our strategic objectives.
If we fail to properly manage our anticipated growth, our business could suffer.
We have been growing steadilymay, in recent periods, prior to the impact of COVID-19. We intend to continue to grow and mayfuture, experience periods of rapid growth and expansion, which could place a significant additional strain on our limited personnel, information technology systems and other resources. In particular, our sales force and distributor network requiresrequire significant management, training, financial and other supporting resources. Any failure by us to manage our growth effectively could have an adverse effect onadversely affect our ability to achieve our development and commercialization goals.
To achieve our long-term revenue goals, we must also will need to successfully increase supply of our products to meet expected customer demand. In the future, we may experience difficulties with yields, quality control, component supply and shortages of qualified personnel, among other problems. These problems could result in delays in product availability and increases in expenses. Any such delay or increased expenseexpenses which could adversely affect our ability to generate revenue.
Future growth will also impose significant added responsibilities on management, including the need to identify, recruit, train and integrate additional employees. In addition, rapid and significant growth will place a strain on our administrative and operational infrastructure.
In order to manage our operations andduring any growth period, we will need to continue to improve our operational and management controls, reporting and information technology systems and financial internal control procedures. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy and our operating results and business could suffer.
We may not be able to strengthen our brand and the brands associated with our products.
We believe that strengthening the Bioventus brand and the brands associated with our products is critical to achieving widespread acceptance of our products, particularly because of the rapidly developing nature of the market for active healing products.products as well as the expansion of our product portfolio due to our recent acquisitions. Promoting and positioning our brand will depend largely on the success of our marketing efforts and the reliability of our products. Historically, our efforts to build our brand have involved marketing expenses, and it is likely that our future marketing efforts will require us to incur additional expenses. These brand promotion activities may not yield increased sales and, even if they do, any sales increases may not offset the expenses we incur to promote our brand and our products. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand and the brands of our products, our products may not be accepted by healthcare providers, which would cause our sales to decrease and would adversely affect our business, results of operations and financial condition.
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing and marketing of our products. This risk exists even if a product is cleared, approved or approvedcertified for commercial sale by the FDA, foreign regulatory authorities or notified bodies and manufactured in facilities regulated by the FDA or an applicable foreign regulatory authority. Our products are designed to affect, and any future products will be designed to affect, important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our products or our products in development could result in patient injury or death. The medical device industry has historically been subject to extensive litigation over product liability claims, and we cannot assure you that we will not face product liability claims. We may be subject to product liability claims if our products or products in development cause, or merely appear to have caused, patient injury or death, even if such injury or death was as a result of supplies or components that are produced by third-party suppliers. Product liability claims may be brought against us by consumers, healthcare providers or others selling or otherwise coming into contact with our products, among others. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in:
•costs of litigation;
•distraction of management’s attention from our primary business;
•the inability to commercialize existing or new products;
•decreased demand for our products or, if cleared or approved, products in development;
•damage to our business reputation;
•product recalls or withdrawals from the market;
•withdrawal of clinical trial participants;
•substantial monetary awards to patients or other claimants; and
•loss of net sales.
While we have attempted and may continue to attempt to manage our product liability exposure by proactively recalling or withdrawing from the market any defective products, any recall or market withdrawal of our products may delay the supply of those products to our customers and may impact our reputation. For example, we have in the past instituted a voluntary recall for certain of our products. We cannot assure you that we will be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future or that these efforts will have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Such recalls and withdrawals may also be used by our competitors to harm our reputation for product safety or be perceived by patients as a safety risk when considering the use of our products, either of which could adversely affect our business, results of operations and financial condition.
In addition, although we have product liability and clinical study liability insurance that we believe is appropriate, this insurance is subject to deductibles and coverage limitations. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, coverage may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at an acceptable cost or on acceptable terms or otherwise protect against potential product liability claims, we could be exposed to significant liabilities. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could adversely affect our business, results of operations and financial condition.
Fluctuations in the demand for our products or our inability to forecast demand accurately may influence the ability of our suppliers to meet our delivery needs or result in excess product inventory.
We are required by some of our contracts with suppliers of our products to forecast future product demand or meet minimum purchase requirements. Our HA product supply agreement for Durolane isagreements are subject to a minimum order volume for each order and purchase amounts arevolumes based in part on forecasts. We are also subject to certainforecasts, annual minimum purchase requirements for GELSYN-3 and SUPARTZ FX and purchase amounts are based on rolling annual forecasts. Our forecasts are based on multiple assumptions of product and market demand, which may cause our estimates to be inaccurate. If we underestimate demand, we may not have adequate supplies and could have reduced control over pricing, availability and delivery schedules with our suppliers, which could prevent us from meeting increased customer or consumer demand and harm our business. However, if we overestimate our demand, we may have underutilized assets and may experience reduced margins. If we do not accurately align our supplies with demand and/or fail to meet contractual minimum purchase requirements, our business, results of operations and financial condition may be adversely affected. For example, if we fail to order the minimum order quantity of SUPARTZ FX from SKK we are obligated to pay SKK a specified fee equal to the number of units needed to meet the minimum order quantity multiplied by a specified percentage of the purchase price.
We may face issues with respect to the supply of our products or their components, including increased costs, disruptions of supply, shortages, contamination or mislabeling.
We are dependent on a limited number of suppliers for our products and components used in the manufacturing process of our products. Our top three suppliers providesingle-source third-party manufacturers supply us with our HA products and components that constituted 53%43%, 54%42% and 49%51% of total net sales for the years ended December 31, 2020, 20192023, 2022 and 2018,2021, respectively. Durolane, GELSYN-3Many of the acquired Bioness and SUPARTZ FXMisonix products are supplied by single-source third-party manufacturers. Our Exogen system undergoes final assembly with components procured from variousalso dependent on a limited number of suppliers including a transducer, which is a key component that is supplied by a single source supplier.for these products and their components. We may not be able to renew or enter into new contracts with our existing suppliers following the expiration of such contracts on commercially reasonable terms, or at all. Additionally, certain of our devices require circuit boards and other electronic components that could be in short supply. The unavailability of such components from our suppliers may impact our ability to meet the customer demand for these products.
In particular, theThe success of our bone graft substitutions product portfolio,certain Surgical products depends on our suppliers continuing to have access to donated human cadaveric tissue, as well as the maintenance of high standards in their processing methodology. The supply of such donors can fluctuate over time. We cannot be certain that our current suppliers who rely on allograft bone, tissue, plus any additional sources that our suppliers identify in the future, will be sufficient to meet our product needs. Our dependence on a limited number of third-party suppliers and the challenges that they may face in obtaining adequate supplies of allograft bone tissue involve several risks, including limited control over pricing, availability, quality and delivery schedules. We may be unable to find an alternative supplier in a reasonable time period or on commercially reasonable terms, if at all, which would adversely affect our business, results of operations and financial condition.
If any of our products or the components used in our products are alleged or proven to include quality or product defects, including as a result of improper methods of tissue recovery from donors and disease transmission from donated tissue or illegal harvesting, we may need to find alternate supplies, delay production of our products, discard or otherwise dispose of our products, or engage in a product recall, all of which may adversely affect our business, results of operations and financial condition. If our products or the components in our products are affected by adverse prices or quality or other concerns, we may not be able to identify alternate sources of components or other supplies that meet our quality controls and standards to sustain our sales volumes or on commercially reasonable terms, or at all.
We rely on a limited number of third-party manufacturers to manufacture certain of our products.
Third-party manufacturers generally manufacture Durolane, GELSYN-3, SUPARTZ FX,our HA products, Exogen components, certain Surgical Solutions products, PNS and our bone graft substitutions product portfolio.rehabilitation devices. We have developed in-house assembly capabilities for our Exogen system. We and our third-party manufacturers are required to comply with the QSR which is a set of FDA regulations that establishes cGMP requirements for medical devices and covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of such devices. Moreover, certain of our products may be re-classified as drugs, and we are planning to seek approval of a product pursuant to the BLA pathway. In each case, such products would be required to comply with the cGMP requirements that apply to drugs and biologics, respectively.
There are a limited number of suppliers and third-party manufacturers that operate under FDA’s QSR requirements and that have the necessary expertise and capacity to manufacture our products or components for our products. As a result, it may be difficult for us to locate manufacturers for our anticipated future needs, and our anticipated growth could strain the ability of our current suppliers and third-party manufacturers to deliver products, materials and components to us. Upon expiration of our existing agreements with these third-party manufacturers, we may not be able to renegotiate the terms of our agreements with these third-party manufacturers on a commercially reasonable basis, or at all.
If we or our third-party manufacturers fail to maintain facilities in accordance with the FDA’s QSR, the noncomplying party could lose the ability to manufacture our products on a commercial scale. Loss of this manufacturing capability would limit our ability to sell some of our products, including Durolane, GELSYN-3, SUPARTZ FX and our bone graft substitutions product portfolio, which are manufactured by single-source third-party manufacturers. See “Business—Manufacturing and supply.”products.
The manufacturing of our products may not be easily transferable to other sites in the event that any of our third-party manufacturers experience breakdown, failure or substandard performance of equipment, disruption of supply or shortages of, or quality issues with, components of our products and other supplies, labor problems, power outages, adverse weather conditions, natural disasters (including natural events caused by or intensified by climate change), global pandemics, such as COVID-19, or the need to comply with environmental and other directives of governmental agencies. From time to time, a third-party manufacturer may experience financial difficulties, bankruptcy or other business disruptions, which could disrupt our supply of finished goods or require that we incur additional expense by providing financial accommodations to the third-party manufacturer or taking other steps to seek to minimize or avoid supply disruption, such as establishing a new third-party manufacturing arrangement with another provider. The loss of any of these third-party manufacturers or the failure for any reason of any of these third-party manufacturers to fulfill their obligations under their agreements with us, including a failure to meet our quality controls and standards, may result in disruptions to our supply of finished goods. We may be unable to locate an additional or alternate third-party manufacturing arrangement that meets our quality controls and standards in a timely manner or on commercially reasonable terms, if at all. If this occurs, our business, results of operations and financial condition will be adversely affected.
If our facilities are damaged or become inoperable, we will be unable to continue to research, develop and manufacture our products and, as a result, our business, results of operations and financial condition may be adversely affected until we are able to secure a new facility.
We do not have redundant facilities for the final assembly of our Exogen system. Our other facilities and equipment would be costly to replace and could require substantial lead-time to repair or replace. Our facilities may be harmed or rendered inoperable by natural or man-made disasters, including, but not limited to, tornadoes, flooding, fire and power outages. Such disasters may render it difficult or impossible to manufacture and commercialize our products and conduct our research and development activities for new products, line extensions and expanded indications. The inability to perform those activities, combined with our limited inventory of supplies, components and finished product, may result in the inability to continue manufacturing or supplying our products during such periods and the loss of customers or harm to our reputation. Although we possess insurance for damage to our facilities and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and this insurance may not continue to be available to us on acceptable terms, or at all.
If we fail to maintain our numerous contractual relationships, our business, results of operations and financial condition could be adversely affected.
We are party to numerous contracts in the normal course of our business, including our supply and distribution agreements for Durolane, which has a current term expiring in December 2115, GELSYN-3, which has a current term expiring in February 2026, and SUPARTZ FX, which has a current term expiring in December 2028.agreements. We have contractual relationships with suppliers, distributors and agents, as well as service providers. In the aggregate, these contractual relationships are necessary for us to operate our business. From time to time, we amend, terminate or negotiate our contracts. We may also periodically be subject to, or make claims of breach of contract, or threaten legal action relating to our contracts. These actions may result in litigation. At any one time, wehave a number of negotiations under way for new or amended commercial agreements. We devote substantial time, effort and expense to the administration and negotiation of contracts involved in our business. However, these contracts may not continue in effect past their current term or we may not be able to negotiate satisfactory contracts in the future with current or new business partners, which may adversely affect our business, results of operations and financial condition.
If we are unable to manage, train, maintain and grow our direct sales team and network of independent distributors, we may not be able to generate anticipated sales or we may be subject to regulatory or enforcement action.
Our operating results are directly dependent upon the sales and marketing efforts of not only our direct sales team, but also our independent distributors. If our direct sales team or independent distributors fail to adequately promote, market and sell our products, our sales could significantly decrease.
We face significant challenges and risks in managing our geographically dispersed distribution network and retaining the individuals who make up that network. If any members of our direct sales team were to leave us, or if any of our independent distributors were to cease to do business with us, our sales could be adversely affected. In such a situation, we may need to seek alternative independent distributors or increase our reliance on our direct sales team, which may not prevent our sales from being adversely affected. If a member of our direct sales team or independent distributor were to depart and be retained by one of our competitors, we may be unable to prevent them from helping competitors solicit business from our existing customers, which could further adversely affect our sales. Because of the competition for their services, we may be unable to recruit or retain additional qualified independent distributors or to hire additional direct sales team members to work with us on favorable or commercially reasonable terms, if at all. Failure to hire or retain qualified members of our direct sales team or independent distributors would prevent us from maintaining or expanding our business and generating sales.
If we launch new products or increase our marketing efforts with respect to existing products, we will need to expand the reach of our marketing and sales networks. Our future success will depend largely on our ability to continue to hire, train, retain and motivate skilled members of our direct sales team and independent distributors with significant technical knowledge in active healing products. New hires require training and take time to achieve full productivity. If we fail to train new hires adequately, or if we experience high turnover in our sales force in the future, we cannot be certain that new hires will become as productive as may be necessary to maintain or increase our sales. Further, if we are unable to adequately train new hires and/or members of our direct sales team, if new hires and/or members of our direct sales team engage in practices such as the promotion of unapproved or off-label uses of our devices or if new hires and/or members of our direct sales team assist with the reimbursement process in a manner that results in false or fraudulent claims for reimbursement being submitted to government or private payers, we may be subject to investigations or regulatory or enforcement actions by governmental authorities or third party payers for reasons such as the promotion of unapproved or off-label uses of our devices, inappropriate actions and involvement in the reimbursement process, or inappropriate completion of reimbursement forms. See “—Risks related to government regulation—We may be subject to enforcement action if we engage in improper claims submission practices and resulting audits or denials of our claims by government agencies could reduce our net sales or profits.”
If we are unable to expand our sales and marketing capabilities domestically and internationally, we may not be able to effectively commercialize our products, which would adversely affect our business, results of operations and financial condition.
Actual or attempted breaches of security, unauthorized access to or disclosure of information, denial of service attackscyberattacks, or other incidents or the perception that personal and/or other sensitive or confidential information in our possession or control or in the possession or control of our third-party vendors or service providers is not secure, could result in a material loss of business, substantial legal liability or significant harm to our reputation.
We receive, collect, process, use and store directly and through third-party vendors and service providers a large amount of information, including personally identifiable information, protected health information and other sensitive and confidential information. This data is often accessed by us through transmissions over public and private networks, including the Internet. The secure transmission of such information over the Internet and other mechanisms is essential to maintain confidence in our Information Technology (IT)information technology systems. Despite the privacy and security measures we have in place to ensure compliancecomply with applicable laws, regulations and contractual requirements, our facilities and systems, and those of our third-party vendors and service providers, are vulnerable to privacy and security incidents including, but not limited to, computer hacking, breaches, acts of vandalism or theft, computer viruses and other malware, including ransomware orand other forms of cyber-attack,cyberattacks, misplaced or lost data, programming and/or human errors, orand other similar events. A party, whether internal or external, that is able to circumvent our security systemsmeasures or those of our third-party vendors and service providers could, among other things, misappropriate or misuse sensitive or confidential information, misappropriate user information or other proprietary information, or cause significant interruptions in our operations. Internal or external parties have and will continue to attempt to circumvent our security systems and those of our vendors and service providers, and we expect that we may in the future continue to experience, among other things, external attacks on our network, and attempts to gain unauthorized access to sensitive and confidential information, such as reconnaissance probes, denial of service attempts, malware attacks, malicious software attacks and phishing attacks.
attacks, such as an external phishing incident that occurred in January 2023, targeting an employee with plausible-sounding prompts to send information to Company leadership. This security incident did not expose protected health information, or affect any of the company’s systems, and was reported to authorities in the relevant regions. Because the techniques used to circumvent security systems can be highly sophisticated and change frequently, and often are not recognized until launched against a target and may originate from less regulated and remote areas around the world, we may be unable to proactively address all possible techniques or implement adequate preventive measures for all situations. Attacks upon information technology systems are also increasing in their frequency, level of persistence, and sophistication, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. We may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. Recent, well-publicized attackscyberattacks on prominent companies have resulted in the theftunauthorized access to and acquisition of significant amounts of sensitive and personalconfidential information and the disruption of important systems and services. These incidents demonstrate the sophistication of the perpetratorsthreat actors and magnitude of the threat posed to companies across the nation, including the health care industry. For example, a third-party vendor recently informed us that Change Healthcare, a subsidiary of UnitedHealth Group that acts as an intermediary for processing certain of our claims for reimbursement related to our Exogen device to commercial payers, experienced an incident in which a cybersecurity threat actor gained access to some of its information technology systems. As a result of the Change Healthcare incident, certain of our patient billing and collections processes have been disrupted. While we have identified an alternative claim processing intermediary and have resumed claims submissions to some payers, this event may cause delays in a portion of our claims submissions to some commercial payers thereby delaying the related cash remittances to us. As of the date of this Annual Report, UnitedHealth Group is still investigating this incident, including any potential impact on claims and patient data. On March 7, 2024, UnitedHealth Group issued a statement indicating that it expects to begin testing and reestablish connectivity to the effected claims network to restore service beginning March 18, 2024. We do not presently believe that the Change Healthcare incident has materially affected, or is reasonably likely to materially affect the Company, including with respect to our claims collection and cash flows. We continue to evaluate the impact of the Change Healthcare incident on our Company.
If someone is able to circumventgain unauthorized access to our systems or breachthose of our security systems,third-party vendors or service providers, they could stealaccess, acquire, or alter any information located therein or cause interruptions to our operations. Security breaches or attemptsand attempted security breaches thereof could also damage our reputation and expose us to a risk of monetary loss and/or litigation, fines, sanctions, and sanctions.reputational damage. We also face risks associated with security breaches affecting third parties that conduct business with us or our customers and others who interact with our data. While we maintain insurance that covers certain security and privacy breaches, we may not carry appropriate insurance or maintain sufficient coverage to compensate for all potential liability. Additionally, the costs incurred to remediate any data security or privacy incident could be substantial.
We cannot assure you that our third-party service providers or service providers with access to our or our customers’, suppliers’, trial patients’, and employees’ personally identifiable and other sensitive or confidential information in relation to which we are responsible will not breach contractual obligations imposed by us, or that they will not experience data security breaches, cyber-attacks or attempts thereof,other incidents negatively impacting the privacy or security of sensitive or confidential information or our service providers’ ability to provide services to us, which could have a corresponding effect on our business including putting us in breach of our obligations under privacy laws and regulations and/or which could in turn adversely affect our business, results of operations and financial condition. While we attempt to address the associated risks by performing security assessments and detailed due diligence, we cannot assure you that these contractual measures and our own privacy and security-related due diligence safeguards will protect us from the risks associated with the third-party processing, storage and transmission of such information.information by service providers and others acting on our behalf.
Failure of a key information technology and communication system, process or site could adversely affect our business, results of operations and financial condition.
We rely extensively on information technology and communication systems and software and hardware products, including those of external providers, to conduct business. These systems and software and hardware impact, among other things, ordering and managing components of our products from suppliers, shipping products to customers on a timely basis, processing transactions, coordinating our sales activities across all of our products, summarizing and reporting results of operations, complying with regulatory, legal or tax requirements, data security and other processes necessary to manage our business.
Despite any precautions we may take, our systems and software and hardware could be exposed to damage or interruption from circumstances beyond our control, such as fire, natural disasters, systems failures, power outages, cyber-attacks, terrorism, energy loss, telecommunications failure, security breaches and attempts thereof, computer viruses and similar disruptions affecting the global Internet. Although we have taken steps to prevent system failures and have back-up systems and procedures to prevent or reduce disruptions, such steps may not prevent an interruption of services and our disaster recovery planning may not be adequate or account for all contingencies. Additionally, our insurance may not adequately compensate us for all losses or failures that may occur. If our systems or software and hardware are damaged or cease to function properly and our business continuity plans do not effectively compensate on a timely basis, we may suffer interruptions in our operations, which could adversely affect our business, results of operations and financial condition.
We will need to improve and upgrade our systems and infrastructure as our operations grow in scale in order to maintain the reliability and integrity of our systems and infrastructure. The expansion of our systems and infrastructure will require us to commit substantial financial, operational and technical resources before the volume of our business increases, with no assurance that the volume of business will increase. Any service outages or delays due to the installation of any new or upgraded technology (and customer issues therewith), or the impact on the reliability of our data from any new or upgraded technology could adversely affect our business, results of operations and financial condition.
The ongoing conflict in Israel could have an adverse impact our business and the extent to which the conflict will impact our future operations and financial condition remains uncertain.
We have a manufacturing facility in Hod Hasharon, Israel which produces certain of our rehabilitation products, including with product components from third-party suppliers located elsewhere in Israel. Together, these products account for less than 10% of our total net sales for the year ended December 31, 2023.
In October 2023, Hamas infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas also launched extensive rocket attacks on the Israeli population and industrial centers located along Israel’s border with the Gaza Strip and in other areas within the State of Israel. Following the attack, Israel’s security cabinet declared war against Hamas. In addition, the clash between Israel and Hezbollah in Lebanon, may escalate in the future into a greater regional conflict.
Hostilities involving Israel could have an adverse impact on our future operations and financial condition. In the event that the Company’s facility in Hod Hasharon is damaged or otherwise materially disrupted as a result of hostilities in Israel, the Company’s ability to manufacture and deliver certain rehabilitation products to its customers could be materially adversely affected.
The conflict in Israel also could cause situations where medical product certifying or auditing bodies cannot visit our manufacturing facility in Israel in order to review relevant certifications or clearances, which could lead to temporary suspensions or even cancellations of our product clearances or certifications. The conflict in Israel could result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements.
Delays and disruptions as related to exports from Israel also may be imposed in the future and adversely impact our ability to manufacture and deliver some rehabilitation products in a timely manner.
In addition, approximately 25 of our employees currently reside in Israel and work at the manufacturing facility in Hod Hasharon. Government-imposed restrictions on movement and travel and other precautions taken to address the ongoing conflict may temporarily disrupt our employees’ ability to effectively perform their daily tasks. The Israel Defense Force (the “IDF”), the national military of Israel, is a conscripted military service, subject to certain exceptions. Some of our employees may be subject to military service in the IDF and may be called to serve. It is possible that there will be further military reserve duty call-ups in the future, which may affect our business due to a shortage of skilled labor at our manufacturing facility in Israel.
The ultimate impact of the ongoing hostilities in Israel on our operations and financial condition remains uncertain and depends on many factors, including, but not limited to, the duration and severity of the conflict in Israel, the extent to which we can successfully rely on our other manufacturing facilities and third-party suppliers with respect to certain of our rehabilitation products and the cost of any necessary mitigation efforts. To the extent the conflict in Israel adversely impacts our business, operations and financial condition, they may also have the effect of heightening many of the other risks described in our SEC filings.
Our business subjects us to economic, political, regulatory and other risks associated with international sales and operations that could adversely affect our business, results of operations and financial condition.
Since we sell our products in many different jurisdictions outside the United States, our business is subject to risks associated with conducting business internationally. We anticipate that net sales from international operations will continue to represent a portion of our total net sales. In addition, a numbersome of our third-party manufacturing facilities and suppliers of our products and product components are located outside the United States.States, including in Israel. Accordingly, our future results could be harmed by a variety of factors associated with international sales and operations, including:
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country;
•customers in some foreign countries potentially having longer payment cycles;
•disadvantagesexposure of competing against companies from countries that are not subjectour foreign operations to liability under U.S. laws and regulations, including the U.S. Foreign Corrupt Practices Act (FCPA)(“FCPA”), regulations of the U.S. Office of Foreign Assets Controls, and U.S. anti-money laundering regulations, as well as exposuredisadvantages of our foreign operationscompeting against companies from countries that are not subject to liability under these regulatory regimes;
•training of third-partiesthird parties on our products and the procedures in which they are used;
•reduced protection for and greater difficulty enforcing our intellectual property rights;
•unexpected changes in tariffs, trade barriers and regulatory requirements, export licensing requirements or other restrictive actions by foreign governments;
•difficulty in staffing and managing widespread operations, including compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•foreign taxes, including withholding of payroll taxes;
•workforce uncertainty in countries where labor unrest is more common than in the United States;
•exposure to liability under a variety of local, national and multinational laws and regulations in multiple jurisdictions, including data privacy laws, healthcare and pharmaceutical laws, antitrust and competition laws, anti-bribery and anti-corruption laws and international trade laws;
•international regulators and third-party payers requiring additional clinical studies prior to approving or allowing reimbursement for our products;
•complexities associated with managing multiple payer reimbursement regimes, government payers or patient self-pay systems;
•production shortages resulting from any events affecting material supply or manufacturing capabilities abroad; and
•business interruptions resulting from geopolitical actions, including war and terrorism (including the current conflicts between Russia and Ukraine and between Israel and Hamas), global pandemics or natural disasters including earthquakes, typhoons,hurricanes, floods and fires. If the current conflicts between Russia and Ukraine and between Israel and Hamas escalate or spill over to or otherwise impacts additional regions, it could heighten many of the other risk factors included in our SEC filings.
In addition, further expansion into new international markets may require significant resources and the efforts and attention of our management and other personnel, which may divert resources from our existing business operations. As we expand our business internationally, our success will depend, in large part, on our ability to anticipate and effectively manage these and other risks associated with our operations outside of the United States.
We are exposed to foreign currency risks, which may adversely affect our business, results of operations and financial condition.
External events such as the withdrawal by the United Kingdom from the EU, global pandemics, the ongoing uncertainty regarding actual and potential shifts in U.S. and foreign trade, economic and other policies and the passage of U.S. taxation reform legislation each have caused, and may continue to cause, significant volatility in currency exchange rates. Because some of our revenue, expenses, assets and liabilities are denominated in foreign currencies, we are subject to exchange rate and currency risks. In preparing ourOur financial statements which are presented in U.S. dollars we must convert all non-U.S. dollar financial results to U.S. dollars at varying exchange rates. Thiswhich may ultimately result in currency gain or loss, the outcome of which we cannot predict. Furthermore, to the extent that we incur expenses or earn revenue in currencies other than in U.S. dollars, any change in the values of those foreign currencies relative to the U.S. dollar could cause our profits to decrease or our products to be less competitive against those of our competitors. To the extent that our current assets denominated in foreign currency are greater or less than our current liabilities denominated in foreign currencies, we face potential foreign exchange exposure.
To minimize such exposures, we have entered, and may in the future enter, into derivative instruments related to forecasted foreign currency transactions or currency hedges from time to time. Losses from changes in the value of the Euro or other foreign currencies relative to the U.S. dollar could adversely affect our business, results of operations and financial condition.
We are subject to differing tax rates in several jurisdictions in which we operate, which may adversely affect our business, results of operations and financial condition.
We will be subject to taxes in the United States and certain foreign jurisdictions. Due to economic and political conditions, tax rates in various jurisdictions, including the United States, may be subject to change. Our future effective tax rates could be affected by changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities and changes in tax laws or their interpretation. In addition, we may be subject to income tax audits by various tax jurisdictions. Although we believe our income tax liabilities are reasonably estimated and accounted for in accordance with applicable laws and principles, an adverse resolution by one or more taxing authorities could have a material impact on the results of our operations
International tariffs applied to goods traded between the United States and China for restrictions on goods imported from certain regions of China may adversely affect our business, results of operations and financial condition.
International tariffs, including tariffs applied to goods traded between the United States and China, may adversely affect our business, results of operations and financial condition. Since the beginning of 2018, there has been increasing rhetoric,discussion, in some cases coupled with legislative or executive action, from several U.S. and foreign leaders regarding the possibility of instituting tariffs against foreign imports of certain materials. More specifically, in March and April of 2018, the U.S. and China applied tariffs to certain of each other’s exports. The institution of trade tariffs both globally and between the U.S. and China specifically carries the risk of adversely affecting overall economic condition, which could have a negative impact on us as imposition of tariffs could cause an increase in the cost of our products and the components for our products, specifically with respect to our Exogen system, which may adversely affect our business, results of operations and financial condition.
The 2019 Credit Agreement contains financial In addition, the U.S. has previously enacted, and operating restrictions that may limit our access to credit. If we fail to comply with financialit or other covenantscountries may in the 2019 Credit Agreement, we may be requiredfuture enact legislation that limits or prohibits the use of foreign manufactured equipment or supplies from China, such as the Uyghur Forced Labor Prevention Act, which imposes a ban on virtually all imports from the Xinjiang region of China unless companies are able to repay indebtedness to our existing lenders,prove that the products were not made with forced labor, which may harm our liquidity.
On December 6, 2019, we entered into a $250.0 million credit and guaranty agreement, or the 2019 Credit Agreement, with Wells Fargo Bank National Association, as administrative agent and collateral agent, and a syndicate of other entities as lenders. As of December 31, 2020, we had outstanding indebtedness of $190.0 million under our term loan (leaving $49.9 million available under our revolving credit facility after giving effect to $0.1 million in an outstanding letter of credit). We are subject to certain covenants under the 2019 Credit Agreement, including, but not limited to:
•a minimum interest coverage ratio and a maximum debt leverage ratio requirement as defined in our credit agreement;
•restrictions on the declaration or payment of certain distributions on or in respect of our equity interests;
•restrictions on acquisitions, investments and certain other payments;
•limitations on the incurrence of new indebtedness;
•limitations on the incurrence of new liens on property or assets;
•limitations on transfers, sales and other dispositions;
•limitations on entering into transactions with affiliates; and
•limitations on making any material change in any of our business objectives that could reasonably beis expected to have a material adverse effect on the repayment of our credit agreement.
Such indebtedness could have significant consequences, including:
•requiring a substantial portion of our cash flows to be dedicated to debt service payments instead of funding growth, working capital, capital expenditures, investments or other cash requirements;
•reducing our flexibility to adjust to changing business conditions or obtain additional financing;
•exposing us to the risk of increased interest rates as certain of our borrowings, including borrowings under our term loan, are at variable rates, making it more difficult for us to make payments on our indebtedness;
•restricting us from making strategic acquisitions or causing us to make non-strategic divestitures;
•subjecting us to restrictive covenants that may limit our flexibility in operating our business; and
•limiting our ability to obtain additional financing for working capital, capital expenditures, debt service requirements and general corporate or other purposes.
In addition, we may not be able to comply with these financial covenants described above in the future. In the absence of a waiver from our lenders, any failure by us to comply with these covenants in the future may result in the declaration of an event of default, which could adversely affect our business, results of operations and financial position. See Part II, Item 7. Management’s discussion and analysis of financial condition and results of operations—Indebtedness.
Uncertainty relating to the LIBOR calculation process and potential phasing out of LIBOR in the future may adversely affect our financing costs.
Currently, the 2019 Credit Agreement utilizes the London Interbank Offered Rate (LIBOR), or various alternative methods set forth in the 2019 Credit Agreement to calculate interest on any borrowings. National and international regulators and law enforcement agencies have conducted investigations into a number of rates or indices known as “reference rates.” Actions by such regulators and law enforcement agencies may result in changes to the manner in which certain reference rates are determined, their discontinuance or the establishment of alternative reference rates. In particular, on July 27, 2017, the Chief Executive of the United Kingdom Financial Conduct Authority (FCA), which regulates LIBOR, announced that the FCA will no longer persuade or compel banks to submit rates for the calculation of LIBOR after 2021 or, in certain cases, 2023, pursuant to an updated announcement in November 2020. Such announcements indicate that the continuation of LIBOR on the current basis cannot and will not be guaranteed after such dates, as applicable, and it appears highly likely that LIBOR will be discontinued or modified by 2021 or, in certain cases 2023.
At this time, it is not possible to predict the effect that these developments, any discontinuance, modification or other reforms to LIBOR or any other reference rate, or the establishment of alternative reference rates may have on LIBOR, other benchmarks or LIBOR-based debt instruments. Uncertainty as to the nature of such potential discontinuance, modification, alternative reference rates or other reforms could cause the interest rates calculated for the 2019 Credit Agreement to be materially different than expected, which could have a material adverse effect on our financing costs.
Due to the high degree of uncertainty regarding the implementation and impact of the CARES Act and other legislation related to COVID-19, there can be no assurance as to the total amount of financial assistance we will receive or that we will be able to comply with the applicable terms and conditions for retaining such assistance.
On March 27, 2020, the Coronavirus Aid, Relief and Economic Security (CARES) Act was signed into law, which is aimed at providing emergency assistance and health care for individuals, families, and businesses affected by the COVID-19 pandemic and generally supporting the U.S. economy. The CARES Act, among other things, includes provisions relating to refundable payroll tax credits, deferment of employer social security payments, net operating loss carryback periods, alternative minimum tax credit refunds, and modifications to the net interest deduction limitations. The CARES Act and similar legislation intended to provide assistance related to the COVID-19 pandemic also authorized $175.0 billion in funding to be distributed by HHS to eligible health care providers. This funding, known as the Provider Relief Fund, is designated to fund eligible healthcare providers’ healthcare-related expenses or lost revenues attributable to COVID-19. On December 27, 2020, the Consolidated Appropriations Act, 2021 was signed into law, which adds $3.0 billion to the Provider Relief Fund. Payments from the Provider Relief Fund are subject to certain eligibility criteria, as well as reporting and auditing requirements, but do not need to be repaid to the U.S. government if recipients comply with the applicable terms and conditions.
In reliance on the CARES Act, we deferred our employer social security payroll tax payments from May 2020 until the remainder of the 2020 calendar year of which, 50% is deferred until December 31, 2021, with the remaining 50% deferred until December 31, 2022. The Company has deferred $1.9 million of payroll tax payments as of December 31, 2020, half of which has been recorded in accrued liabilities and the remainder in other long-term liabilities on the condensed consolidated balance sheet. We are in the process of analyzing other provision of the CARES Act to determine the financial impact on our condensed consolidated financial statements.
In April 2020, we received, without request, a $1.2 million payment from the Provider Relief Fund from HHS. We determined that we complied with the conditions to be able to keep and use the funds as reimbursement for health care related expenses and lost revenue attributable to the public health emergency resulting from COVID-19 and submitted to HHS the required attestation to agree to the applicable terms and conditions of the Provider Relief Fund Phase I General Distribution. In July 2020, we applied for and received a second Provider Relief Fund payment totaling $2.9 million, which is subject to the same conditions as the initial payment. The payments were recorded as other income on the condensed consolidated statement of operations and comprehensive income (loss) for year ended December 31, 2020.
Due to the high degree of uncertainty regarding the implementation of the CARES Act, the Consolidated Appropriations Act, 2021 and other stimulus legislation, there can be no assurance that the terms and conditions of the Provider Relief Fund or other relief programs will not change or be interpreted in ways that affect our ability to comply with such terms and conditions in the future, which could affectconduct our ability to retain such assistance. We will continue to monitor our compliance with the terms and conditions of the Provider Relief Fund, including demonstrating that the distributions received have been used for healthcare-related expenses or lost revenue attributable to COVID-19. If we are unable to comply with current or future terms and conditions, our ability to retain some or all of the distributions received may be impacted, and we may be subject to actions including payment recoupment, audits and inquiries by governmental authorities, and criminal, civil or administrative penalties.
Our future capital needs are uncertain and we may need to raise additional funds in the future, and such funds may not be available on acceptable terms or at all.
We believe that our current cash and cash equivalents, in combination with the borrowing availability under our credit facilitybusiness and our expected cash from operations, will be sufficient to meet our projected operating requirements for the foreseeable future. However, we may seek additional funds from public and private stock offerings, borrowings under our existing or new credit facilities or other sources in order to fund future initiatives related to the expansion of our business, which financing may not be available on acceptable or commercially reasonable terms, if at all. For example, pursuant to the Option and Equity Purchase Agreement with CartiHeal and its shareholders, CartiHeal has a put option that would require us to purchase 100% of CartiHeal’s shares for $350.0 million under certain conditions, or the Put Option. The Put Option is only exercisable by CartiHeal upon pivotal clinical trial success of the Agili-C device and we may terminate the Put Option at any time ending 30 days after receipt by CartiHeal of the statistical report regarding the final results of the pivotal clinical upon payment of $30.0 million to CartiHeal. See Part II, Item 7. Management’s discussion and analysis of financial condition and results of operations—Strategic transactions—CartiHeal (developer of Agili-C) investment and option and equity purchase agreement.operations.
Furthermore, if we issue equity or debt securities to raise additional capital, our existing stockholders may experience dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional capital through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our products, potential products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise capital on acceptable terms, we may not be able to develop or enhance our products, execute our business plan, take advantage of future opportunities, or respond to competitive pressures, changes in our supplier relationships, or unanticipated customer requirements. Any of these events could adversely affect our business, results of operations and financial condition.
Risks related to government regulation
The risk factors listed below describe the risks we face related to government regulation. The companies who manufacture or produce certain of the products we distribute face similar risks with respect to government regulation relating to such products. If such suppliers are unable to comply with government regulations, they may not be able to continue to supply us with products, which could adversely affect our business, results of operations and financial condition.
Our products and operations are subject to extensive governmental regulation, and our failure to comply with applicable requirements could cause our business to suffer.
The healthcare industry, and in particular the medical device industry, are regulated extensively by governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies and authorities. The FDA and other U.S. and foreign governmental agencies and authorities regulate and oversee, among other things:
•design, development and manufacturing;
•testing, labeling, content and language of instructions for use and storage;
•clinical trials;
•product safety;
•marketing, sales and distribution;
•premarket clearance, approval and approval;certification;
•conformity assessment procedures;
•record-keeping procedures;
•advertising and promotion;
•recalls and other field safety corrective actions;
•postmarketpost market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury;
•postmarketpost market studies; and
•product import and export.
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
The failure to comply with applicable regulations could jeopardize our ability to sell our products and result in enforcement actions such as:
•administrative or judicially imposed sanctions;
•unanticipated expenditures to address or defend such actions;
•injunctions, consent decrees or the imposition of civil penalties or fines;
•recall or seizure of our products;
•total or partial suspension of production or distribution;
•refusal to grant pending or future clearances, approvals or approvalscertifications for our products;
•withdrawal or suspension of regulatory clearances, approvals or approvals;certifications;
•clinical holds;
•untitled letters or warning letters;
•refusal to permit the import or export of our products; and
•criminal prosecution of us or our employees.
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and harm our reputation, business, results of operations and financial condition.
Moreover, governmental authorities outside the United States have become increasingly stringent in their regulation of medical devices, and our products may become subject to more rigorous regulation by non U.S.non-U.S. governmental authorities in the future. U.S. or non-U.S. government regulations may be imposed in the future that adversely affect our business, results of operations and financial condition. The European Commission has harmonized national regulations for the control of medical devices through European Medical Device DirectivesRegulations with which manufacturers must comply. Under these new regulations, manufacturing plants must have received a full Quality Assurance Certification from a “Notified Body” in order to be able to sell products within the member states of the EU. This certification allows manufacturers to stamp the products of certified plants with a “CE” mark. Products covered by European Commission regulations that do not bear the CE mark cannot be sold or distributed within the EU. We have receivedRefer to Part I, Item 1A. Risk Factors—Risks related to government regulation—Regulatory reforms, such as the EU Medical Devices Regulation, could limit our ability to market and distribute our products after clearance, approval or certification is obtained and make it more difficult or costly for allus to obtain regulatory clearance, approval or certification of any future products, which could adversely affect our manufacturing facilities.competitive position and materially affect our business and financial results.
We may be subject to enforcement action if we engage in improper claims submission practices and resulting audits or denials of our claims by government agencies could reduce our net sales or profits.profits and could lead to significant civil or criminal penalties and other liability.
In connection with our Exogen system, we submit claims directly to, and receive payments directly from, the Medicare and Medicaid programs and private payers. Therefore, we are subject to extensive government regulation, including detailed requirements for submitting claims under appropriate codes and maintaining certain documentation, including evidence that all medical necessity requirements are met to support our claims. Billing for our Exogen system is complex, time-consuming and expensive, particularly for items and services provided to government healthcare program beneficiaries, such as Medicare and Medicaid. Reimbursement claims may be adversely affected by improper completion of the CMNCertificates of Medical Necessity (“CMN”) required in connection with Medicare claims for the Exogen system and we may be subject to investigations by governmental authorities or third partythird-party payers and required to prove the validity of the claims or the authenticity of the signatures on the CMNs under investigation. Reimbursement claims may also be adversely affected by the promotion of our devices for unapproved or off-label uses or assistance with the reimbursement process that could result in false or fraudulent claims for reimbursement being submitted to government or private payers. Depending on the billing arrangement and applicable law, we bill various payers, all of which may have different prior authorization, patient qualification and medical necessity requirements, as well as patients for any applicable co-payments or co-insurance amounts. In addition, we may also face increased risk in our collection efforts, including potential write-offs of doubtful accounts and long collection cycles, any of which could adversely affect our business, results of operations and financial condition.
We are also required to implement compliance procedures and oversight,to oversee, train and monitor our employees,employees’ compliance with those procedures, appeal coverage and payment denials, and perform internal audits periodically to assess compliance with applicable laws and regulations as well as internal compliance policies and procedures. We are required to report and return any overpayments received from government payers within 60 days of identification and exercise of reasonable diligence to investigate credible information regarding potential overpayments. Failure to identify and return such overpayments exposes the provider or supplier to liability under federal false claims laws. For example, in February 2021 we entered into a settlement agreement with the United States Attorney’s Office for the Middle District of North Carolina and the Office of Inspector General of the U.S. Department of Health and Human Services (“OIG”) to resolve potential liabilities associated with a self-disclosure we made to the OIG in November 2018 regarding violations of certain Medicare claim submission requirements. See Part I, Item 1A. Risk Factors—Factors—Risks related to government regulation—We are subject to federal, state and foreign laws and regulations relating to our healthcare business, and could face substantial penalties if we are determined not to have fully complied with such laws, which would adversely affect our business, results of operations and financial condition. Moreover, Medicare contractors and state Medicaid agencies periodically conduct pre- and post-payment reviews and other audits of claims and are under increasing pressure to more closely scrutinize healthcare claims and supporting documentation. We may be subject to prepayment and post-payment reviews, as well as audits of claims in the future. Private payers may from time to time conduct similar reviews and audits. Any third-party payer reviews and audits of our claims could result in material delays in payment, material recoupments, overpayments, claim denials, fines, revocations of billing privileges, bars on re-enrollment in federal or state healthcare programs, cancellation of our agreements or damage to our reputation, any of which would reduce our net sales and profitability.
For example, in July of 2018 we became aware of allegations that certain of our sales personnel may have been completing Section B of the CMN required in connection with Medicare claims for the Exogen system, which, under federal law, must be completed by the physician and/or physician staff. Together with our outside counsel, Ropes & Gray LLP, we initiated an investigation into these allegations, and we determined that the CMN forms for a portion of Medicare claims for the Exogen system were in fact improperly completed by our sales representatives, some of which also failed to meet CMS coverage requirements. As a result of our findings, we made a self-disclosure on November 30, 2018 to the OIG, under the Provider Self-Disclosure Protocol. Our self-disclosure disclosed the extent of our findings relating to the inappropriate completion of CMN forms by our sales personnel and offered to make repayment for such claims which failed to meet CMS coverage requirements and which we submitted to the Medicare program between October 1, 2012 and September 30, 2018, the statutory period applicable to such conduct. The total value of impacted claims was $30.1 million in the aggregate. In October 2019, our outside counsel received a letter from the Office of the United States Attorney in the Middle District of North Carolina (USAO), stating that the USAO would be working with the OIG to resolve our self-disclosure. After settlement discussions with the USAO and OIG, on January 25, 2021 we reached a settlement agreement with the USAO and the OIG with respect to the submission of Medicare claims that did not meet CMS coverage requirements and for which our sales representatives completed Section B of the CMN forms. On February 22, 2021, we finalized all terms related to the settlement and entered into a formal settlement agreement with the USAO and OIG consistent with our previous agreement in principle and which included releases from associated False Claims Act liability and further Civil Monetary Penalties that are customary in self-disclosures of this type. Under the agreement, we resolved the potential liability related to our self-disclosure for $3.6 million, of which $2.4 million had already been paid through our 2019 return of overpayments described previously, leaving a net payment to be made of $1.2 million. We made payment of the $1.2 million net settlement amount due under the agreement on February 23, 2021. The settlement amount noted above was recorded in the consolidated financial statements for the year ended December 31, 2020. In connection with this settlement, we were not subjected to any non-monetary penalties, such as monitoring agreements or requirements to conduct audits and submit reports to the HHS.
The FDA regulatory process is expensive, time-consuming and uncertain, and the failure to obtain and maintain required regulatory clearances and approvals could prevent us from commercializing our products.
Before we can market or sell a new medical device or other product or a new use of or a claim for or significant modification to an existing medical device in the United States, we must obtain either clearance from the FDA under 510(k) pathway or approval of a PMA,Pre-Market Approval (“PMA”), unless an exemption applies. In the United States, we have obtained 510(k) premarket clearance from the FDA to market certain of our products such as Signafuse Bioactive Bone Graft Putty, Interface Bioactive Bone Graft and Signafuse Mineralized Collagen Scaffold. Our OA joint pain treatment and joint preservationPain Treatment products, including Durolane, GELSYN-3 and SUPARTZ FX, and our Exogen system, have an obtained PMA approval.PMA. In the 510(k) clearance process, before a device may be marketed, the FDA must determine that a proposed device is “substantially equivalent” to a legally-marketed “predicate” device, which includes a device that has been previously cleared through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (preamendments device), a device that was originally on the U.S. market pursuant to an approved PMA application and later downclassified, or a 510(k)-exemptpredicate device. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence. In the PMA process, the FDA must determine that a proposed product is safe and effective for our intended use based, in part, on extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for products that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices.
Modifications to products that are approved through a PMA application generally require FDA approval. Similarly, certain modifications made to products cleared through a 510(k) may require a new 510(k) clearance.
Both the PMA approval and the 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to twelve months, but can last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from six to 18eighteen months, or even longer, from the time the application is filed with the FDA. In addition, a PMA generally requires the performance of one or more clinical trials. Despite the time, effort and cost, we cannot assure you that any particular device will be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory approvals could harm our business.
Any modification to one of our 510(k) cleared products that would constitute a major change in its intended use, or any change that could significantly affect the safety or effectiveness of the device would require us to obtain a new 510(k) marketing clearance and may even, in some circumstances, require the submission of a PMA application, if the change raises complex or novel scientific issues or the product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer’s decision. We may make changes to our 510(k)-cleared products in the future that we may determine do not require a new 510(k) clearance or PMA approval. If the FDA disagrees with our decision not to seek a new 510(k) or PMA approval for changes or modifications to existing devices and requires new clearances or approvals, we may be required to recall and stop marketing our products as modified, which could require us to redesign our products, conduct clinical trials to support any modifications, and pay significant regulatory fines or penalties. If there is any delay or failure in obtaining required clearances or approvals or if the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, our ability to introduce new or enhanced products in a timely manner would be adversely affected, which in turn would result in delayed or no realization of revenue from such product enhancements or new products and could also result in substantial additional costs which could decrease our profitability.
The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:
•if we may not be ableare unable to demonstrate to the FDA’s satisfaction that the product or modification is substantially equivalent to the proposed predicate device or safe and effective for its intended use;
•if the data from our preclinical studies and clinical trials may be insufficient to support clearance or approval, where required; and
•if the manufacturing process or facilities we use may not meet applicable requirements.
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared or approved products on a timely basis. Even after clearance or approval for our products is obtained, we and the products are subject to extensive postmarketpost market regulation by the FDA, including with respect to advertising, marketing, labeling, manufacturing, distribution, import, export, and clinical evaluation. For example, as a condition of approving a PMA application, the FDA may require some form of post-approval study or post-market surveillance, whereby the applicant conducts a follow-up study or follows certain patient groups for a number of years and makes periodic reports to the FDA on the clinical status of those patients when necessary to protect the public health or to provide additional safety and effectiveness data for the device. The product labeling must be updated and submitted in a PMA supplement once results, including any adverse event data from the post-approval study, become available. Failure to conduct post-approval studies in compliance with applicable regulations or to timely complete required post-approval studies or comply with other post-approval requirements could result in withdrawal of approval of the PMA, which would harm our business.
We are also required to timely file various reports with regulatory agencies. If these reports are not timely filed, regulators may impose sanctions and sales of our products may suffer, and we may be subject to product liability or regulatory enforcement actions, all of which could harm our business. In addition, if we initiate a correction or removal for one of our devices, issue a safety alert, or undertake a field action or recall to reduce a risk to health posed by the device, we may be required to submit a report to the FDA, and in many cases, to other regulatory agencies. Such reports could lead to increased scrutiny by the FDA, other international regulatory agencies and our customers regarding the quality and safety of our devices and to negative publicity, including FDA alerts, press releases, or administrative or judicial actions. Furthermore, the submission of these reports has been and could be used by competitors against us in competitive situations and cause customers to delay purchase decisions or cancel orders, which would harm our reputation and business.
The FDA, state and stateforeign authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA, state or stateforeign regulatory agencies, which may include any of the following sanctions:
•adverse publicity, warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties;
•repair, replacement, refunds, recalls, termination of distribution, administrative detention or seizures of our products;
•operating restrictions, partial suspension or total shutdown of production;
•customer notifications or repair, replacement or refunds;
•refusing our requests for 510(k) clearance or PMA approvals or foreign regulatory approvals of new products, new intended uses or modifications to existing products;
•withdrawals of current 510(k) clearances or PMAs or foreign regulatory approvals, resulting in prohibitions on sales of our products;
•FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; and
•criminal prosecution.
Any of these sanctions could also result in higher than anticipated costs or lower than anticipated sales and adversely affect our business, results of operations and financial condition.
In addition, the FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance as a result of a changing regulatory landscape, we may lose any marketing approvals or clearances that we have already obtained or fail to obtain new marketing approvals or clearances, and we may not be able to achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. The results of the 2020 Presidential election may impact our business and industry. Moreover, the Trump administration took several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rule making, issuance of guidance, and review and approval of marketing applications. It is difficult to predict whether or how these executive actions, including the Executive Orders, will be implemented, or whether they will be rescinded or replaced under the Biden administration. The policies and priorities of an incoming administration are unknown and could materially impact the regulatory framework governing our products.
Once obtained, we cannot guarantee that FDA or international product approvals will not be withdrawn or rescinded or that relevant regulatory authorities will not require other corrective action, and any withdrawal, rescission or corrective action could materially affect our business and financial results.
Once obtained, marketing approval can be withdrawn by the FDA or comparable foreign regulatory authorities for a number of reasons, including the failure to comply with ongoing regulatory requirements or the occurrence of unforeseen problems following initial approval. Regulatory authorities could also limit or prevent the manufacture or distribution of our products. Any regulatory limitations on the use of our products or any withdrawal, suspension or rescission of approval by the FDA or a comparable foreign regulatory authority could have a material adverse effect on our business, financial condition, and results of operations.
Legislative or regulatory reforms, including those currently under consideration by FDA and the EU, could make it more difficult or costly for us to obtain regulatory clearance, approval or approvalcertification of any future products and to manufacture, market and distribute our products after clearance, approval or approvalcertification is obtained, which could adversely affect our competitive position and materially affect our business and financial results.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions and regulations governing the regulatory clearance or approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, propose new reclassification orders, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to market or modify our currently cleared products on a timely basis. FDA and foreign regulations depend heavily on administrative interpretation, and we cannot assure you that future interpretations made by the FDA or other regulatory bodies, with possible retroactive effect, will not adversely affect us. Additionally, any changes, whether in interpretation or substance, in existing regulations or policies, or any future adoption of new regulations or policies by relevant regulatory bodies, could prevent or delay approval of our products. In the event our future, or current, products, including HA generally, are classified, or re-classified, as human drugs, combination products, or biologics by the FDA or an applicable international regulatory body, the applicable review process related to such products is typically substantially longer and substantially more expensive than the review process to which they are currently subject as medical devices. In 2018, FDA publicly indicated its intent to consider HA products for certain indications as drugs and has indicated that sponsors of HA products who submit PMAs or PMA supplements for changes in indications for use, formulation or route of administration should obtain an informal or formal classification and jurisdictional determination through a pre-request for determination or request for determination prior to submission. There exists uncertainty with respect to the final interpretation, implementation, and consequences of this development, and this or any other potential regulatory changes in approach or interpretation similar in substance to those mentioned in this paragraph and affecting our products could materially impact our competitive position, business, and financial results.
Moreover,For example, over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products. For example, in November 2018, FDA officials announced forthcoming steps that the FDA intendsintended to take to modernize the 510(k) premarket notification pathway, under Section 510(k) of the FDCA. Among other things, the FDA announced that it planned to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates. These proposals includedincluding plans to potentially sunset certain older devices that were used as predicates under the 510(k) clearance pathway, and to potentially publish a list of devices that have been cleared onpathway. In September 2019, the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. The FDA also announced that it intended to finalizeissued revised final guidance to establishestablishing a premarket review pathway“Safety and Performance Based Pathway” for “manufacturers of certain well-understood device types” as an alternative to the 510(k) clearance pathway and that such premarket review pathway would allowallowing manufacturers to rely on objective safety and performance criteria recognized by the FDA to demonstrate substantial equivalence, obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process.
In May 2019, the The FDA solicited public feedback on its plans to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates, including whether the FDA should publishhas developed and maintains a list of devicesdevice types appropriate for the “safety and performance based” pathway and continues to develop product-specific guidance documents that have been cleared onidentify the basisperformance criteria and recommended testing methodologies for each such device type, where feasible. Some of demonstrated substantial equivalence to predicate devices that are more than 10 years old. The FDA requested public feedback on whether it should consider certain actions that might require new authority, such as whether to sunset certain older devices that were used as predicates under the 510(k) clearance pathway. Thesethese proposals have not yet been finalized or adopted, and the FDA announced that it would seek public feedback prior to publication of any such proposals, and may work with Congress to implement such proposals through legislation. Accordingly, it is unclear the extent to which any proposals, if adopted,changes could impose additional regulatory requirements on us that could delay our ability to obtain new 510(k) clearances, increase the costs of compliance, or restrict our ability to maintain our current clearances, or otherwise create competition that may negatively affect our business.
More recently, in September 2019, the FDA finalized the aforementioned guidance to describe an optional “safety and performance based” premarket review pathway for manufacturers of “certain, well-understood device types” to demonstrate substantial equivalence under the 510(k) clearance pathway, by demonstrating that such device meets objective safety and performance criteria established by the FDA, obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process. The FDA intends to maintain a list device types appropriate for the “safety and performance based pathway” and develop product-specific guidance documents that identify the performance criteria for each such device type, as well as the testing methods recommended in the guidance, where feasible. The FDA may establish performance criteria for classes of devices for which we or our competitors seek or currently have received clearance, and it is unclear the extent to which such performance standards, if established, could impact our ability to obtain new 510(k) clearances, or otherwise create competition that may negatively affect our business.
In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain clearance or approval for, manufacture, market or distribute our products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:require additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping. Additionally, the implementation
Table of the new EU MDR set to take full effect on May 26, 2021 after a one-year postponement due to the COVID-19 pandemic, is expected to change several aspects of the existing regulatory framework in Europe. Specifically, the EU MDR will require changes in the clinical evidence required for medical devices, post-market clinical follow-up evidence, annual reporting of safety information for Class III products, and bi-annual reporting for Class II products, Unique Device Identification (UDI) for all products, submission of core data elements to a European UDI database prior to placement of a device on the market, reclassification of medical devices, and multiple other labeling changes. While we will be able to continue marketing our currently CE-marked products in the EEA after the EU MDR enters into full effect and until the associated CE mark certificates expire, acquiring approvals for new products or renewing our existing CE mark certificates once these expire could be more challenging and costly.Contents Our HCT/P products are subject to extensive government regulation and our failure to comply with these requirements could cause our business to suffer.
In the United States, we sell human tissue-derived BGSs, such as PureBone and OsteoAMP,Surgical Solutions products, which are referred to by the FDA as human cells, tissues and cellular or tissue-based products or (“HCT/Ps.Ps”). In the U.S.,United States, we are marketing our HCT/Ps pursuant to Section 361 of the PHSA and 21 CFR Part 1271 of FDA’s regulations. We do not manufacture these HCT/P products, but serve as a distributor for them. So-called Section 361 HCT/Ps are not currently subject to the FDA requirements to obtain marketing authorizations as long as they meet certain criteria provided in FDA’s regulations. HCT/Ps regulated as “361 HCT/Ps” are currently subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, cGTP, when processing, storing, labeling and distributing HCT/Ps, including required labeling information, stringent record keeping and adverse event reporting. If we or our suppliers fail to comply with these requirements, we could be subject to FDA enforcement action, including, for example, warning letters, fines, injunctions, product recalls or seizures, and, in the most serious cases, criminal penalties. To be regulated as Section 361 HCT/Ps, these products must meet FDA’s criteria to be considered “minimally manipulated” and intended for “homologous use,” among other requirements. HCT/Ps that do not meet the criteria to be considered Section 361 HCT/Ps are subject to the FDA’s regulatory requirements applicable to medical devices, biologics or drugs. Device, biologic or drug HCT/Ps must comply both with the requirements exclusively applicable to Section 361 HCT/Ps and, in addition, with other requirements, including requirements for marketing authorization. For example, Section 361 HCT/Ps do not requireauthorization, such as 510(k) clearance or PMA approval, approval of aor BLA or other premarket authorization from FDAapprovals before marketing. Except as described below with regard to MOTYS, weWe believe our HCT/Ps are regulated solely under Section 361 of the PHSA, and therefore, we have not sought or obtained 510(k) clearance, PMA approval, or licensure through a BLA for such HCT/Ps.
The FDA could disagree with our determination that these human tissue products are Section 361 HCT/Ps and could determine that these products are biologics requiring a BLA or medical devices requiring 510(k) clearance or PMA approval, and could require that we cease marketing such products and/or recall them pending appropriate clearance, approval or licensure from the FDA. For example, the FDA’s CDRH issued us a letter in March 2016 in which it asserted that OsteoAMP meets the definition of a medical device, and requested that we provide CDRH with information in support of our position that OsteoAMP does not require 510(k) clearance or PMA approval. We provided CDRH with the requested information in support of this position in May 2016 and we have received no further inquiries to date. We believe that CDRH’s assertion is unfounded and inconsistent with a 2011 letter from the FDA concluding that OsteoAMP meets the criteria for regulation solely as a Section 361 HCT/P. However, if the FDA were to disagree, and if we are otherwise unsuccessful in asserting our position, the FDA may then require that we obtain 510(k) clearance or PMA approval and that we cease marketing OsteoAMP and/or recall OsteoAMP unless and until we receive clearance or approval. If we have to cease marketing and/or have to recall any of our BGSsSurgical Solutions products including OsteoAmp, our net sales would decrease, which would adversely affect our business, results of operations and financial condition.
HCT/Ps that do not meet the criteria of Section 361 are regulated under Section 351 of the PHSA. Unlike Section 361 HCT/Ps, HCT/Ps regulated as “351”“Section 351” HCT/Ps are subject to premarket review and approval by the FDA. In November 2017, the FDA released a guidance document entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue—Based Products: Minimal Manipulation and Homologous Use—Guidance for Industry and Food and Drug Administration Staff.” The guidance outlined the FDA’s position that all lyophilized amniotic products are more than minimally manipulated and would therefore require a BLA to be lawfully marketed in the United States. The guidance also indicated that the FDA would exerciseresumed enforcement discretion, using a risk-based approach,of IND and premarket approval requirements with respect to the IND application and pre-market approval requirements for certain HCT/Ps for a periodthese products as of 36 months from the issuance date of the guidance to allow manufacturers to pursue its IND application. Under this approach, FDA indicated that high-risk products and uses could be subject to immediate enforcement action; FDA has not clearly stated what must happen by the end of its enforcement discretion period in order to avoid enforcement (i.e., whether a BLA must be approved by that time, or merely submitted). In July 2020, the FDA extended its period of enforcement discretion to May 31,June 1, 2021.
We plan to market MOTYS under the FDA’s policy of enforcement discretion as we pursue marketing authorization under a BLA for the product. We may be required to cease selling MOTYS if the FDA changes the scope of its enforcement discretion or changes the criteria used to assess which products qualify. In addition, following the period of enforcement discretion articulated in FDA’s guidance, we may be required to cease selling MOTYS until such time as we obtain BLA approval or be subject to another enforcement action or penalties. We may also be subject to enforcement on the grounds that we are marketing a product at the same time we are investigating that product pursuant to an IND, in violation of FDA’s prohibition on the preapproval promotion of an investigational product. The loss of our ability to market and sell this product could have an adverse impact on our business, results of operations and financial condition. In addition, we expect the cost to manufacture our products will be higher than our other HCT/Ps because of the costs to comply with the more stringent requirements that apply to products regulated as biologics for which a BLA is required (and not just as Section 361 HCT/Ps). These requirements include satisfying cGMP manufacturing standards and performing ongoing product testing. If we do receive BLA approval for this product, changes such as adding new indications, manufacturing changes and additional labeling claims, will be subject to further testing requirements and FDA review and approval.
In addition, the FDA may in the future modify the scope of its enforcement discretion with respect to Section 361 HCT/Ps or change its position on which current or future products qualify as Section 361 HCT/Ps, or determine that some or all of our HCT/P products may not be lawfully marketed under the FDA’s policy of enforcement discretion. Any regulatory changes could have adverse consequences for us and make it more difficult or expensive for us to conduct our business by requiring pre-market clearance or approval and compliance with additional post-market regulatory requirements with respect to those products.
If clinical studies of our future products do not produce results necessary to support regulatory clearance, approval or approvalcertification in the United States or elsewhere, we will be unable to expand the indications for or commercialize these products.
We will likely need to conduct additional clinical studies in the future to support new indications for our products or for clearances, approvals or approvalscertifications of new product lines, or for the approval or certification of the use of our products in some foreign countries. Clinical testing can take many years, can be expensive and carries uncertain outcomes. The initiation and completion of any of these studies may be prevented, delayed, or halted for numerous reasons. Conducting successful clinical studies requires the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population, the nature of the trial protocol, the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators and support staff, proximity of patients to clinical sites, patient ability to meet the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of our products or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competitive products. In addition, patients participating in clinical trials may die before completion of the trial or suffer adverse medical events unrelated to investigational products.
For example, in late 2017 we began enrollment for the B.O.N.E.S. clinical study, a uniquely designed trial to further broaden the label
Clinical failure can occur at any stage of testing. Our clinical studies may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and non-clinical studies in addition to those we have planned. In addition, failure to adequately demonstrate the safety and efficacy of any of our devices would prevent receipt of regulatory clearance, approval or approvalcertification and, ultimately, the commercialization of that device or indication for use. Even if our future products are cleared in the United States, commercialization of our products in foreign countries would require approval or certification by regulatory authorities or notified bodies in those countries. Approval and certification procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials. Any of these occurrences could adversely affect our business, results of operations and financial condition.
Interim, “top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim, “top-line” or preliminary data from our clinical trials. Interim, top-line, or preliminary data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary, “top-line,” or interim data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim, “top-line,” and preliminary data should be viewed with caution until the final data are available. Differences between preliminary, interim, or “top-line” data and final data could significantly harm our business prospects and may cause the trading price of our common stock to fluctuate significantly.
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our business in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product candidate or our business. If the interim, “top-line,” or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for and commercialize our product candidates, our business, operating results, prospects or financial condition may be harmed.
We may be subject to enforcement action if we engage in improper marketing or promotion of our products, and the misuse or off-label use of our products may harm our image in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines and/or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
The medical devicesCertain products that we currently market have been cleared, approved or approvedcertified by the FDA and other foreign regulatory authorities and notified bodies for specific treatments. However, weWe cannot prevent a physician from using our products outside of such cleared or approved indications for use, known as off-label uses, when in the physician’s independent professional medical judgment, he or she deems it appropriate, anduses. While we do not analyze the ordering practices of physicians with respect to off-label uses. In cases where prescriptionsuses, we are aware of certain off-label uses of our Exogen system are written for off-label uses,EXOGEN and StimRouter products. As a result, we could be subject to regulatory or enforcement actions if we wereare determined to have engaged in promotion of our products for off-label uses, or otherwise determined to have made false or misleading statements about our products. There may be increased risk of injury to patients if physicians attempt to use our products off-label. Furthermore, the use of our products for indications other than those cleared, approved or approvedcertified by the FDA or any foreign regulatory authority or notified body may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
In addition, physicians may misuse our products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk ofliability risks to us for product liability.and medical malpractice related claims. If our products are misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Product liability and related claims could divert management’s attention from our core business, be expensive to defend and result in sizeable damage awards against us that may not be covered by insurance.
Further, our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of off-label use. If the FDA or any foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, the FDA could request that we modify our training, promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. Such enforcement actions may include, but are not limited to, criminal, civil and administrative penalties, treble damages, fines, disgorgement, exclusion from participation in government healthcare programs, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations.
Our products may cause or contribute to adverse medical events that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could materially harm our business.
Some of our marketed products are subject to MDRmedical device reporting obligations, which require that we report to the FDA any incident in which our products may have caused or contributed to a death or serious injury, or in which our products malfunctioned and, if the malfunction were to recur, it could likely cause or contribute to a death or serious injury. The timing of our obligation to report under the MDR regulations is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our products. If we fail to comply with our reporting obligations, the FDA could take action including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device clearances, seizure of our products, or delay in clearance of future products.
We and our third-party manufacturers and suppliers are subject to various governmental regulations related to the manufacturing of our products.
Our products and the manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for such products, will be subject to continued regulatory review, oversight and periodic inspection by the FDA and other domestic and foreign regulatory bodies. In particular, the methods used in, and the facilities used for, the manufacture of the products that we own and distribute that are regulated as medical devices must comply with the FDA’s QSR,Quality System Regulation (“QSR”), which covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of medical devices. The FDA enforces the QSR through periodic announced or unannounced inspections of manufacturing facilities, and both we and our third-party manufacturers and suppliers are subject to such inspections. Similarly, the devices we distribute on behalf of third-party manufacturers that are regulated as Section 361 HCT/Ps must be manufactured in compliance with FDA’s cGTP requirements and other related requirements. Moreover, should any of our HA products be re-classified as drugs, such products would be required to comply with a different set of manufacturing requirements under FDA’s cGMP requirements for drugs. Similarly, if we are successful in obtaining BLA approval for MOTYS, that product will need to comply with the cGMP requirements for biologics, instead of the cGTP requirements that will apply to the product upon our planned launch of the product as a Section 361 HCT/P. The need to comply with different manufacturing requirements may require us to seek new suppliers.
Failure to comply with applicable FDA requirements, or later discovery of previously unknown problems with our products or the manufacturing processes of our third-party manufacturers and suppliers, including any failure to take satisfactory corrective action in response to an adverse regulatory inspection, can result in, among other things:
•administrative or judicially imposed sanctions;
•injunctions or the imposition of civil penalties or fines;
•recall or seizure of our products;
•total or partial suspension of production or distribution;
•refusal to grant pending or future clearances, approvals or approvalscertifications for our products;
•withdrawal or suspension of regulatory clearances, approvals or approvals;certifications;
•clinical holds;
•untitled letters or warning letters;
•refusal to permit the import or export of our products; and
•criminal prosecution of us or our employees.
Any of these actions could prevent or delay us from marketing, distributing or selling our products and would likely harm our business. Furthermore, our suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Our products may be subject to product recalls. A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our products, could adversely affect us.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized drugs, devices and similar products in the event of material deficiencies or defects in their design or manufacture. TheFor example, the FDA’s authority to require a recall for medical devices must be based on a finding that there is reasonable probability that the device would cause serious injury or death. WeIn addition, we have in the past and may alsoin the future decide to voluntarily recall our products if certain deficiencies are found. We haveFor example, in the past instituted a voluntary recall for certain of our products, andDecember 2020 we are currently undertakingundertook a voluntary Class II recall of certain vials of ultrasound gel that we provide with our Exogen system due to particulates, which were microbial in nature, found in the gel. The gel iswas manufactured by a third-party supplier, and we have discontinued the use of that suppliers’ gel and have replaced that gel with that of another manufacturer. We have identified the affected lotsmanufacturer and have notified patients to discard gel bottles from thoseaffected lots. A government-mandated or voluntary recall could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and could adversely affect our reputation and business, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be subject to liability claims, be required to bear other costs, or take other actions that could adversely affect our business, results of operations and financial condition.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA.FDA or foreign regulatory authorities. We may initiate voluntary recalls or corrections for our products in the future that we determine do not require notification of the FDA.FDA or foreign regulatory authorities. If the FDA disagreesor foreign regulatory authorities disagree with our determinations, they could require us to report those actions as recalls and we may be subject to enforcement action.
As we conduct clinical studies designed to generate long-term data on some of our existing products, the data we generate may not be consistent with our existing data and may demonstrate less favorable safety or efficacy. Data we generate may ultimately not be favorable, or could even hurt the commercial prospects for our products.
We are currently collecting and plan to continue collecting long-term clinical data regarding the quality, safety and effectiveness of some of our existing products. The clinical data collected and generated as part of these studies will further strengthen our clinical evaluation concerning safety and performance of these products. We believe that this additional data will help with the marketing of our products by providing surgeons and physicians with additional confidence in their long-term safety and efficacy. If the results of these clinical studies are negative, these results could reduce demand for our products and significantly reduce our ability to achieve expected net sales. We do not expect to undertake such studies for all of our products and will only do so in the future where we anticipate the benefits will outweigh the costs and risks. For these reasons, surgeonsSurgeons and physicians could be less likely to purchase our products than competing products for which longer-term clinical data are available. Also, we may not choose or be able to generate the comparative data that some of our competitors have or are generating and we may be subject to greater regulatory and product liability risks. If we are unable to or unwilling to collect sufficient long-term clinical data supporting the quality, safety and effectiveness of our existing products, our business, results of operations and financial condition could be adversely affected.
We may rely on third parties to conduct our clinical studies and to assist us with preclinical development and if they fail to perform as contractually required or expected, we may not be able to obtain regulatory clearance, approval or approvalcertification to commercialize our products.
We have relied upon and may continue to rely upon third parties, such as contract research organizations (“CROs”), medical institutions, clinical investigators and contract laboratories to assist in conducting our clinical studies, which must be conducted in accordance with applicable regulations, including GCP and our preclinical development activities. We rely on these parties for execution of our studies, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our clinical studies is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards, and our reliance on these third parties does not relieve us of our regulatory responsibilities. GCPs are regulations and guidelines enforced by the FDA and other regulatory authorities for products in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators, trial sites, and CROs. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under applicable manufacturing requirements.
If these third parties fail to successfully carry out their contractual duties, comply with applicable regulatory obligations, including GCP requirements, or meet expected deadlines, or if these third parties must be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to clinical protocols or applicable regulatory requirements or for other reasons, our pre-clinical development activities or clinical studies may be extended, delayed, suspended or terminated. Under these circumstances we may not be able to obtain regulatory clearance, approval or approvalcertification for, or successfully commercialize, our products on a timely basis, if at all, and our business, results of operations and financial condition may be adversely affected.
If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative third parties or to do so on commercially reasonable terms. In addition, our third partiesthird-party contractors are not our employees, and except for remedies available to us under our agreements with them, we cannot control whether or not they devote sufficient time and resources to our on-going clinical, nonclinical and preclinical programs. Switching or adding additional third partiesthird-party contractors involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new contract research organization (CRO)CRO or other third partythird-party vendor commences work. As a result, delays occur, which can materially impact our ability to meet our desired development timelines. Though we carefully manage our relationships with our third partythird-party vendors, including CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
Healthcare regulatory reform may affect our ability to sell our products profitably and could adversely affect our business, results of operations and financial condition.
In the United States and in certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the regulatory and healthcare systems in ways that could prevent or delay marketing approval or certification of our products in development, restrict or regulate post-approval or certification activities of our products and impact our ability to sell our products profitably. In the United States in recent years, new legislation has been proposed and adopted at the federal and state level that is effecting major changes in the healthcare system. In addition, new regulations and interpretations of existing healthcare statutes and regulations are frequently adopted.
In March 2010,By way of example, the Affordable Care Act was signed into law. While the goal of healthcare reform is to expand coverage to more individuals, it also involves increased government price controls, additional regulatory mandates and other measures designed to constrain medical costs. The Affordable Care Act(“ACA”) substantially changeschanged the way healthcare is financed by both governmental and private insurers, encourages improvements in the quality of healthcare items and services and significantly impacts the medical device industry. Among other things, the Affordable Care Act:ACA:
•increased the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;
•created a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
•extended manufacturers’ Medicaid rebate liability to individuals enrolled in Medicaid managed care organizations;
•expanded eligibility criteria for Medicaid programs;
•established a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; and
•implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the Affordable Care Act, as well as effortsACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the former Trump administration to repeal or replace certain aspectsconstitutionality of the ACA. Prior to the Supreme Court's decision, President Biden issued an executive order to initiate a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace, from February 15, 2021 through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. Efforts to reform the marketplace for healthcare services is ongoing, and we expect such challenges and amendments to continue. For example, the Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, ruled that the individual mandate is a critical and inseverable feature of the Affordable Care Act, and therefore, because it was repealed as part of the U.S. Tax Act, the remaining provisions of the Affordable Care Act are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the Affordable Care Act are invalid as well. On March 2, 2020, the United States Supreme Court granted the petitions for writs of certiorari to review this case, and the Court held oral argument on November 10, 2020. The case is expected to be decided in mid-2021. It is unclear how this decision and other efforts to challenge, repeal or replace the Affordable Care Act will impact the Affordable Care Act or our business. We expect there will be additional challenges and amendments to the Affordable Care Act in the future.
The results of the 2020 U.S. presidential and congressional elections have created regulatory uncertainty, including with respect to the U.S. government’s role, in the U.S. healthcare industry. As a result of such elections, there are renewed and reinvigorated calls for health insurance reform, which could cause significant uncertainty in the U.S. healthcare market, could increase our costs, decrease our revenues or inhibit our ability to sell our products. We cannot predict with certainty what impact any U.S. federal and state health reforms will have on us, but such changes could impose new and/or more stringent regulatory requirements on our activities or result in reduced reimbursement for our products, any of which could adversely affect our business, results of operations and financial condition.
In addition, third-party payers regularly update payments to physiciansother legislative changes have been proposed and hospitals where our products are used. For example,adopted since the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) ended the use of the Sustainable Growth Rate Formula, and provided for a 0.5% annual increase in payment rates under the Medicare Physician Fee Schedule through 2019, but no annual update from 2020 through 2025. MACRA also introduced a merit based incentive bonus program for Medicare physicians beginning in 2019. At this time, it is unclear how the introduction of the merit based incentive program will impact overall physician reimbursement under the Medicare program. In addition, theACA was enacted. The Budget Control Act of 2011, imposed reductions toamong other things, reduced Medicare payments to providers ofby 2% per fiscal year, which went into effecteffective on April 1, 2013. Subsequent2013 and, due to subsequent legislative amendments related to the COVID-19 pandemic suspended this Medicare sequestration payment reductionstatute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020, through March 31, 2021, but extended sequestration through 2030. In January 2013, President Obama signed into law2022, unless additional Congressional action is taken. Additionally, the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Third-party payers also regularly update payments to physicians and hospitals where our products are used. By way of example, the Medicare Access and CHIP Reauthorization Act of 2015, or MACRA, enacted on April 16, 2015, repealed the formula by which Medicare made annual payment adjustments to physicians and replaced the former formula with fixed annual updates and a new system of incentive payments that are based on various performance measures and physicians’ participation in alternative payment models such as accountable care organizations. Legislative and regulatory reforms and executive actions intended to reduce the costs of prescription drugs and medical devices are also ongoing in the United States and abroad. It is unclear what effect new quality and payment programs, such as MACRA, may have on our business, financial condition, results of operations or cash flows. These and other payment updates could directly impact the demand for our products or any products we may develop in the future, if cleared or approved.
We expect that the Affordable Care Act, as well as other healthcare reform measures that may be adopted in the future, maycould result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and in additional downward pressure on the price that we receive for any cleared or approved products. Furthermore, we believe that many individuals who have obtained insurance coverage through the health insurance exchanges which arose as a result of the Affordable Care ActACA have done so with policies that have significantly higher deductibles than policies they may have obtained prior to its enactment. Because the out-of-pocket costs of undergoing certain procedures for patients who have not met their deductible for a given year would be significantly higher than they historically would have been, these patients may be discouraged from undergoing certain procedures due to the cost. Any reluctance on the part of patients to undergo procedures utilizing our products due to cost could impact our ability to expand sales of our products and could adversely impact our business, results of operations and financial condition.
We are subject to federal, state and foreign laws and regulations relating to our healthcare business, and could face substantial penalties if we are determined not to have fully complied with such laws, which would adversely affect our business, results of operations and financial condition.
Both inIn our capacity as a pharmaceutical and medical device manufacturer, and/or as a supplier of covered items and services to federal health care program beneficiaries, and with respect to which items and services for which we submit claims for reimbursement from such programs, we are subject to healthcare fraud, waste and abuse regulation and enforcement by federal, state and foreign governments, which could adversely impact our business, results of operations and financial condition. Healthcare fraud and abuse and health information privacy and security laws potentially applicable to our operations include:
•the federal Anti-Kickback Statute (“AKS”), which applies to our marketing practices, educational programs, pricingprohibits the knowing and discounting policies and relationships with healthcare providers, by prohibiting, amongwillful offer, payment, solicitation or receipt of any bribe, kickback, rebate or other things, persons and entities from knowingly and willfully soliciting, receiving, offering or providing remuneration intended to induce or reward, orfor referring an individual, in return for eitherordering, leasing, purchasing or recommending or arranging for or to induce the referral of an individual for, or the purchase, orderordering, purchasing or recommendationleasing of an itemitems or service reimbursable under aservices covered, in whole or in part, by any federal healthcare program, such as the Medicare or Medicaid programs.and Medicaid. A person or entity does not need to have actual knowledge of thisthe statute or specific intent to violate it to have committed a violation. Violations are also subjectPenalties for violating the AKS include civil penalties of up to civil monetary$120,816 per violation plus three times the amount of the improper remuneration, criminal penalties up to $100,000 for eachper violation, plus up to three times the remuneration involved. Civil penalties for such conduct can further be assessed under the federal False Claims Act. Violations of the federal Anti-Kickback Statute may also result civil and criminal penalties, including criminal fines of up to $100,000 and imprisonmentprison terms of up to ten years, orand exclusion from Medicare, Medicaidparticipation in the Federal health care programs. Under the Civil Monetary Penalties statute, physicians who pay or other governmental programs;accept kickbacks also face penalties of up to $50,000 per kickback plus three times the amount of the prohibited remuneration;
•the “Starkfederal physician self-referral law, the Stark Law,” which, subject to certain enumerated statutory and regulatory exceptions, prohibits a physicianphysicians from referring Medicare or Medicaid patients to an entity providing “designatedfor the provision of certain designated health services,” or “DHS”, which includes durableboth prescription drugs and medical equipment,devices, if the physician or a member of such physician’s immediate family memberhas a direct or indirect financial relationship (including an ownership interest or a compensation arrangement) with the entity, and prohibits the entity from billing Medicare or Medicaid for such DHS. In addition to reimbursing the government any associated overpayment, violations of the physician, has an ownership or investment interestStark Law can lead to: (1) civil penalties of nearly $29,000 per claim (in 2023, adjusted annually for inflation); (2) three times the amount of damages suffered by the government; and (3) potential exclusion from participation in or compensation arrangement with such entity that does not comply with the requirementsfederal healthcare programs;
•the federal civil and criminal false claims laws, including the False Claims Act, or “FCA”, which imposeimposes civil and criminal penalties through governmental, civil whistleblower or qui tam actions, againstliability on individuals or entities for, among other things,that knowingly presenting,submit false or causing to be presented,fraudulent claims for payment or approval to the federal government that are false or fraudulent, knowingly makingmake, or cause to be made, a false statement materialin order to an obligation to payhave a false claim paid, including qui tam or transmit money or property to the federal government or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay or transmit money or property to the federal government. Suits filed under the False Claims Act, can be brought by any individual on behalfwhistleblower suits. Penalties for a violation of the government, known as ‘‘qui tam’’ actions, and such individuals, commonly known as ‘‘whistleblowers,’’ may share in any amounts paid by the entityFCA include fines up to the government in fines or settlement. The frequency of filing qui tam actions has increased significantly in recent years, causing greater numbers of pharmaceutical, medical device and other healthcare companies to have to defend a False Claims Act action. When an entity is determined to have violated the False Claims Act, the government may impose civil fines and penalties ranging from $11,665 to $23,331$27,894 for each false claim, plus trebleup to three times the amount of damages and excludecaused by each false claim. In addition, the entity from participation in Medicare, Medicaid and other federal healthcare programs. The government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback StatuteAKS or Stark Law constitutes a false or fraudulent claim for purposes of the false claims statutes;FCA;
•the federal civil monetary penalties laws,beneficiary inducement provisions of the Civil Monetary Penalties Law, which impose civil fines for, among other things, theprohibits, an individual or entity from offering or transfer of remuneration to a Medicare or statefederal healthcare program beneficiary ifthat the personindividual or entity knows or should know it is likely to influence the beneficiary’s selection ofbeneficiary to order or receive healthcare items or services from a particular provider, practitioner,provider. Violations of the CMPL may result in administrative penalties ranging from $5,000 to $100,000 per violation depending on the conduct involved;
•the criminal healthcare fraud provisions of Health Insurance Portability and Accountability Act, or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies;
•HIPAA“HIPAA”, and its implementing regulations, which created federal criminal lawsrelated rules that prohibit among other things,knowingly and willfully executing or attempting to execute a scheme or artifice to defraud any healthcare benefit program or falsifying, concealing or covering up a material fact or making any material false, statements relating tofictitious or fraudulent statement in connection with the delivery of or payment for healthcare matters.benefits, items or services. Similar to the federal Anti-Kickback Statute,AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation;
•HIPAA, as amended by HITECH, and its implementing regulations, which also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of protected health information, or PHI;
•the federal Physician Payments Sunshine Act, which requires certain applicable manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions)federal healthcare programs, to monitor and report annually to the government information related toCMS, certain payments orand other “transferstransfers of value” madevalue to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other healthcare providers (physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists, anesthesiology assistants and certified nurse midwives) and teaching hospitals, and beginning in 2022, physician assistants, nurse practitioners,applicable manufacturers and other practitioners, and requires applicable manufacturersgroup purchasing organizations, to report annually to the government ownership and investment interests held by the providers described abovesuch physicians and their immediate family members and payments or other “transfers of value” to such provider owners. Failure to submit required information may result in civilmembers. Civil monetary penalties of $11,766 per failure up to an aggregate of $176,495 per year (or up$1,000,000 as adjusted annually may be imposed on reporting entities if they fail to an aggregate of $1.177 million per year for “knowing failures”), for all payments, transfers of valuereport information in a timely, accurate or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission, and may result in liability under other federal laws or regulations;complete manner;
•federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;customers;
•federal government price reporting laws, which require us to calculatelaws; and report complex pricing metrics in an accurate and timely manner to government programs, and where the failure to report such prices may expose us to potential liability; and
•analogous state and foreign law equivalents of each of the above federal laws, and regulations, such asstate anti-kickback self-referral, fee-splitting and false claims laws that may apply to items or services reimbursed by any third-party payer, including commercial insurers;laws; state laws that require pharmaceutical andrequiring device companies to comply with the industry’s voluntaryspecific compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise thatstandards, restrict payments that may be made to healthcare providers; state laws that require drugproviders and device manufacturers toother potential referral sources, and report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing information;expenditures; and state and foreign laws governing the privacy and security of certain health information, such as GDPR, which imposes obligations and restrictions on the collection and use of personal data relatingrelated to individuals locatedinsurance fraud in the EU (including health data), manycase of which differ from each other in significant ways and some of which may be more stringent than HIPAA or HITECH.claims involving private insurers.
The risk of us being found in violation of these laws and regulations is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. We are unable to predict what additional federal, state or foreign legislation or regulatory initiatives may be enacted in the future regarding our business or the healthcare industry in general, or what effect such legislation or regulations may have on us. Federal, state or foreign governments may impose additional restrictions or adopt interpretations of existing laws that could adversely affect us.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities, including certain sales and marketing practices and financial arrangements with physicians and other healthcare providers, some of whom recommend, use, prescribe or purchase our products, and other customers, could be subject to challenge under one or more of such laws. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to substantial penalties, including administrative, civil and criminal penalties, damages, fines, additional reporting requirements and oversight if we become subject to a Corporate Integrity Agreement or similar agreement to resolve allegations of non-compliance with these laws, exclusion from governmental healthcare programs, disgorgement and related overpayment obligations, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely impact our business, results of operations and financial condition.
In 2018, we identified non-compliance with certain U.S. federal statutes and requirements governing the Medicare program in related to improper completion of Certificate for Medical Necessity (“CMN”) forms. In November 2018, we made a voluntary self-disclosure related to this matter to the Office of Inspector General of the U.S. Department of Health and Human Services (“OIG”) pursuant to the OIG’s Provider Self-Disclosure Protocol. After settlement discussions with the Office of the United States Attorney in the Middle District of North Carolina (“USAO”) and OIG, on February 22, 2021, we entered into a formal settlement agreement, which included releases from associated False Claims Act liability and further Civil Monetary Penalties that are customary in self-disclosures of this type, and agreed to pay $3.6 million in resolution of this matter.We are subject to governmental regulation and other legal obligations, particularly related to privacy, data protection and information security, and we are subject to consumer protection laws that regulate our marketing practices and prohibit unfair or deceptive acts or practices. Our actual or perceived failure to comply with such obligations could harm our business.
We are subject to diverse laws and regulations relating to data privacy and data security, including, in the United States, HIPAA and, in the EU, EEA, Regulation 2016/679, known as the GDPR. New privacy ruleslaws and regulations are being enacted in the United States, particularly at the state level, and globally, and existing ones are being updated and strengthened. Complying with these numerous, complex and often changing regulations is expensive and difficult,difficult. We strive to comply with all applicable laws and other legal obligations relating to privacy, data security, and data protection. However, given that the scope, interpretation, and application of these laws and regulations are often uncertain and may be conflicting, it is possible that these obligations may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another and/or in a manner that conflicts with our practices. Failure or perceived failure by us or our service providers to comply with any privacy, data security, or data protection laws or data security lawsother obligations, or any security incident or data breach involving the misappropriation, loss or other unauthorized use or disclosure of sensitive or confidential patient or consumer information, whetherexperienced by us, one of our business associatesservice providers, or another third-party,party, could adversely affect our business, results of operations and financial condition, includingbusiness. Such impacts include but are not limited to: investigation costs, materiallegal fees, fines and penalties; compensatory, special, punitive, and statutory damages; enforcement actions; litigation; reputational damage; consent orders regarding our privacy and security practices; requirements that we provide consumer notices, credit monitoring services and/or credit restoration services or other relevant services to individuals impacted individuals;by a data breach; adverse actions against our licenses to do business; and injunctive relief. Furthermore,
In the United States, HIPAA, as amended, and regulations implemented thereunder (collectively referred to as “HIPAA”) imposes, among other things, certain standards relating to the privacy, security, transmission and breach reporting of protected health information (“PHI”) on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their business associates that perform certain services that generally involve creating, receiving, maintaining or transmitting PHI for or on behalf of such covered entities, and their covered subcontractors. HIPAA requires covered entities, such as us, as well as business associates to develop and maintain administrative, physical and technical safeguards to protect PHI, and meet certain notification requirements in the event of a breach of unsecured PHI.
Additionally, under HIPAA, covered entities must report breaches of unsecured PHI to affected individuals without unreasonable delay, not to exceed 60 days following discovery of the breach or, if earlier, the date on which the breach would have been discovered through the exercise of reasonable diligence. Notification also must be made to the U.S. Department of Health and Human Services Office for Civil Rights and, in certain circumstances, to the media. Business associates must report breaches of unsecured PHI to covered entities within 60 days of discovery of the breach by the business associate or its agents or, if earlier, the date on which the breach would have been discovered through the exercise of reasonable diligence. All U.S. states, the District of Columbia, Guam, Puerto Rico, and the U.S. Virgin Islands have enacted data breach notification laws. Some of these rulesbreach notification laws impose notification obligations that are constantly changing.in addition to, or inconsistent with, the HIPAA Breach Notification Rule, which can present compliance challenges.
Entities that are found to be in violation of HIPAA, which may occur in connection with, among other things, a breach of unsecured PHI, a complaint about privacy practices or an audit by the U.S. Department of Health and Human Services (“HHS”), may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. The HHS Office for Civil Rights actively enforces HIPAA and frequently issues significant fines and penalties. HIPAA also authorizes state Attorneys General to file suit on behalf of residents of their states. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
U.S. states have adopted various privacy and security laws and regulations, some of which may be more stringent than HIPAA. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our future customers and strategic partners. For example, the CCPA took effect on January 1, 2020. The CCPA establishes a new privacy framework for covered businesses and provides new and enhanced dataCalifornia Consumer Privacy Act (“CCPA”) creates individual privacy rights tofor California residents such as affording consumersand increases the right to accessprivacy and delete their information and to opt outsecurity obligations of entities handling certain sharing and sales of personal information. The CCPA imposes severe statutory damagesprovides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal information. This private right of action is expected to increase the likelihood of, and risks associated with, data breach litigation. Further, the California Privacy Rights Act (“CPRA”), which amended the CCPA, imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt-out rights for certain uses of sensitive data. It remains unclear how various provisionsalso created the California Privacy Protection Agency, which is authorized to issue substantive regulations and enforce law. Additional states, including Colorado, Connecticut, Delaware, Florida, Indiana, Iowa, Montana, Oregon, Tennessee, Texas, Utah and Virginia have enacted similar comprehensive privacy laws. Other states, including Nevada and Washington, have recently enacted robust health privacy laws. Legislation has been proposed in other states and at the federal level, reflecting a trend toward more stringent privacy legislation in the United States. These developments are likely to result in increased privacy and data security enforcement. Additional compliance investment and potential business process changes may be required, and the enactment of new laws could have potentially conflicting requirements that would make compliance challenging and burdensome.
The Federal Trade Commission (“FTC”) and many state Attorneys General also continue to enforce federal and state consumer protection laws against companies for online collection, use, dissemination and security practices that appear to be unfair or deceptive. For example, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure can constitute unfair acts or practices in or affecting commerce in violation of Section 5(a) of the CCPA willFTC Act. The FTC expects a company’s data security measures to be interpretedreasonable and enforced. The CCPA contains an exemption for medicalappropriate in light of the sensitivity and volume of consumer information governed byit holds, the CMIAsize and for PHI collected by a covered entity orcomplexity of its business, associate governed byand the privacy,cost of available tools to improve security and breach notification rules established pursuantreduce vulnerabilities. In addition to HIPAA, butenforcing against organizations, the precise application and scopeFTC has made clear that it may seek to hold officers personally liable for privacy or security violations of this exemption is not yet clear, andtheir organizations, having done so in the law may still apply to certain aspectspast.
In Europe, the GDPR imposes strict requirements for processing the personal data of our business. The CCPA may lead other states to pass comparable legislation, with potentially greater penalties, and more rigorous compliance requirements relevant to our business, andindividuals within the EEA. Companies that may not include exemptions for businesses subject to HIPAA. The effects of the CCPA, and other similar state or federal laws, are potentially significant and may require us to modify our data processing practices and policies and to incur substantial costs and potential liability in an effort tomust comply with such legislation.
The privacy laws in the EU have been significantly reformed. On May 25, 2018, the GDPR entered into forceface increased compliance obligations and became directly applicable in all EU member states. The GDPR implementsrisk, including more stringent operational requirements than its predecessor legislation. For example, the GDPR requires us to make more detailed disclosures to data subjects, requires disclosure of the legal basis on which we can process personal data, makes it harder for us to obtain valid consent for processing, requires the appointmentrobust regulatory enforcement of data protection officers when sensitive personal data, such as health data, is processed on a large scale, provides more robust rightsrequirements and potential fines for data subjects, introduces mandatory data breach notification through the EU, imposes additional obligations on us when contracting with service providers and requires us to adopt appropriate privacy governance including policies, procedures, training and data audit. If we do not comply with our obligations under the GDPR, we could be exposed to finesnoncompliance of up to the greater of €20 million or up to 4% of our totalthe annual global annual revenue in the event of a significant breach. In addition, we may be the subject of litigation and/or adverse publicity, which could adversely affect our business, results of operations and financial condition.
Prior to the effectivenessrevenues of the noncompliant company, whichever is greater. Among other requirements, the GDPR the US-EU Safe Harbor framework provided a method which permitted the transferregulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States, and the efficacy and longevity of current transfer mechanisms between the EU and the United States remains uncertain. For example, in 2016, the EU and United States agreed to a transfer framework for data transferred from the EU to the United States, under European privacy law; in 2015 it was declared invalid and replaced withcalled the US-EU Privacy Shield framework, or Privacy Shield. OnIn July 16, 2020, the Court of Justice of the European Union, or CJEU, invalidatedEU (“CJEU”) limited how organizations could lawfully transfer personal data from the EU/EEA to the United States by invalidating the Privacy Shield. WhileShield for purposes of international transfers and imposing further restrictions on the CJEU upheld the adequacyuse of the standard contractual clauses (a standard form(“SCCs”). The European Commission issued revised SCCs on June 4, 2021 to account for the decision of contract approvedthe CJEU and recommendations made by the European Commission as an adequate personalData Protection Board (“EDPB”). The United States and the EU recently agreed to a new data transfer mechanism and potential alternative to replace the Privacy Shield), it made clear that reliance on them aloneShield known as the EU-U.S. Data Privacy Framework, which may not necessarily be sufficient in all circumstances; this has created increasing uncertainty. This recent development will require ussubject to review and amend the legal mechanisms by which we make and/or receive personal data transfers to/in the U.S.challenges. As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the standard contractual clausesSCCs cannot be used, and/or start takingand take additional enforcement action,actions, we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer personal data between and among countries and regions in which we operate, it could affect the manner in which we provide our services, the geographical location or segregation of our relevant systems and operations, and could adversely affect our financial results.
Additionally, starting onfrom January 1, 2021, (followingfollowing the United Kingdom’s departure from the EU),EU, we will have had to comply with the GDPR and the UK GDPR (i.e., the GDPR as implemented into UK law) if we offer services to UK users, monitor their behavior or are established in the United Kingdom.. Failure to comply with the UK GDPR can result in fines up to the greater of £17£17.5 million (approximately $20$21 million), or 4% of global revenue. However, the relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear. For example, it is unclear what the roleThe European Commission has adopted an adequacy decision in favor of the Information Commissioner’s Office will be following the end of the transitional period. In addition, it is likely that documentation will need to be put in place between UK entities and entities in EuropeanUnited Kingdom, enabling data transfers from EU member states to ensure adequate safeguards arethe United Kingdom without additional safeguards. However, the UK adequacy decision will automatically expire in place for data transfers, which may result in increased costs with respect to transfers of personal data betweenJune 2025 unless the European Union and the UK, which would increase our expenses. We may find it necessary or advantageous to join industry bodies or self-regulatory organizationsCommission extends that impose stricter compliance requirements than those set out in applicable laws, including the GDPR. We may also be bound by contractual restrictions that prevent us from participating in data processing activities that would otherwise be permissible under applicable laws, including the GDPR. Such strategic choices may impact our ability to use and exploit data, and may have an adverse impact on our business.decision.
Failure to comply with the FCPA and laws associated with our activities outside the United States could adversely affect our business, results of operations and financial condition.
We are subject to the FCPA and other anti-bribery legislation around the world. The FCPA generally prohibits covered entities and their intermediaries from engaging in bribery or making other prohibited payments, offers or promises to foreign officials for the purpose of obtaining or retaining business or other advantages. In addition, the FCPA imposes recordkeeping and internal controls requirements on publicly traded corporations and their foreign affiliates, which are intended, to, among other things, to prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made. As we conduct our business in jurisdictions outside of the United States, we face significant risks if we fail to comply with the FCPA and other laws that prohibit improper payments, offers or promises of payment to foreign governments and their officials and political parties by us and other business entities for the purpose of obtaining or retaining business or other advantages. In many foreign countries, particularly in countries with developing economies, it may be a local custom that businesses operating in such countries engage in business practices that are prohibited by the FCPA or other laws and regulations. Although we have implemented a company policy requiring our employees and consultants to comply with the FCPA and similar laws, such policy may not be effective at preventing all potential FCPA or other violations. Although our agreements with our international distributors clearly state our expectations for our distributors’ compliance with U.S. laws, including the FCPA, and provide us with various remedies upon any non-compliance, including the ability to terminate the agreement, we also cannot guarantee our distributors’ compliance with U.S. laws, including the FCPA. Therefore, there can be no assurance that our employees and agents, or those companies to which we outsource certain of our business operations, have not and will not take actions that violate our policies or applicable laws, for which we may be ultimately held responsible. Any violation of the FCPA, other anti-bribery legislation, including the UK Bribery Act and the Brazil Clean Company Act, or related policies could result in severe criminal or civil sanctions, which could adversely affect our business, results of operations and financial condition.
Furthermore, we are subject to the export controls and economic embargo rules and regulations of the United States, including, but not limited to, the Export Administration Regulations and trade sanctions against embargoed countries, which are administered by the Office of Foreign Assets Control within the Department of the Treasury, as well as the laws and regulations administered by the Department of Commerce.Commerce and the Department of State. These regulations limit our ability to market, sell, distribute or otherwise transfer our products or technology to prohibited countries or persons.persons, or for prohibited end-uses. A determination that we have failed to comply, whether knowingly or inadvertently, may result in substantial penalties, including fines, enforcement actions, civil and/or criminal sanctions, the disgorgement of profits, the imposition of a court-appointed monitor, as well as the denial of export privileges, and may adversely affect our business, results of operations and financial condition.
If we fail to meet Medicare accreditation and surety bond requirements or DMEPOS supplier standards, it could adversely affect our business, results of operations and financial condition.
Our Exogen system is classified by CMS and third-party payers as durable medical equipment. Suppliers of Medicare durable medical equipment, prosthetics, orthotics and supplies (DMEPOS)(“DMEPOS”) must be accredited by an approved accreditation organization as meeting DMEPOS quality standards adopted by CMS and are required to meet surety bond requirements. In addition, Medicare DMEPOS suppliers must comply with Medicare supplier standards in order to obtain and retain billing privileges, including meeting all applicable federal and state licensure and regulatory requirements. CMS periodically expands or otherwise clarifies the Medicare DMEPOS supplier standards, and states periodically change licensure requirements, including licensure rules imposing more stringent requirements on out-of-state DMEPOS suppliers. We believe we are currently in compliance with these requirements. If we fail to maintain our Medicare accreditation status and/or do not comply with Medicare surety bond or supplier standard requirements or state licensure requirements in the future, or if these requirements are changed or expanded, it could adversely affect our business, results of operations and financial condition.
Our operations involve the use of hazardous and toxic materials, and we must comply with environmental, health and safety laws and regulations, which can be expensive, and could adversely affect our business, results of operations and financial condition.
We are subject to a variety of federal, state, local and foreign laws and regulations relating to the protection of the environment or of human health and safety, including laws pertaining to the use, handling, storage, disposal and human exposure to hazardous and toxic materials. Liability under environmental laws can be imposed on a joint and several basis (which could result in an entity paying more than its fair share) and without regard to comparative fault, and environmental laws are likely to become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations, which could adversely affect our business, results of operations and financial condition.
Our employees, independent distributors, independent contractors, suppliers and other third parties may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could expose us to liability and hurt our reputation.
We are exposed to the risk that our employees, independent distributors, independent contractors, suppliers and others may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (1) FDA laws and regulations, including those laws that require the reporting of true, complete and accurate information to the FDA, (2) manufacturing standards, (3) healthcare fraud and abuse laws, or (4) laws that require the true, complete and accurate reporting of financial, billing, and claims information or data. Activities subject to these laws also involve the improper use or misrepresentation of information obtained in the course of clinical trials, creating fraudulent data in our preclinical studies or clinical trials or illegal misappropriation of product, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred.
If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and financial results, including, without limitation, the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our business, results of operations and financial condition.
Risks relatedRegulatory reforms, such as the EU Medical Devices Regulation, could limit our ability to intellectual property mattersmarket and distribute our products after clearance, approval or certification is obtained and make it more difficult or costly for us to obtain regulatory clearance, approval or certification of any future products, which could adversely affect our competitive position and materially affect our business and financial results.
The risk factors listed below describeEU Medical Devices Regulation, which became effective in May 2021, was adopted with the risksaim of ensuring better protection of public health and patient safety. Among other things, the EU Medical Devices Regulation (“MDR”) imposed changes in the clinical evidence for medical devices, post-market clinical follow-up evidence, annual reporting of safety information for Class III products, and bi-annual reporting for Class II products, Unique Device Identification (“UDI”) for all products, submission of core data elements to a European UDI database prior to placement of a device on the market, reclassification of medical devices, and multiple other labeling changes.
While we face related to intellectual property matters. The companies who own certain of the products we distribute face similar risks with respect to intellectual property relating to such products. If such suppliers are unable to protect their intellectual property rights, they may not be able to continue marketing our currently CE-marked products in the Europe after the effective date of the EU MDR until the associated CE mark certificates expire, securing renewals of our existing CE mark certificates to allow for continued marketing of the product after CE mark expiration or obtaining certifications for new products requires the performance of certain conformity assessment procedures by a notified body. Notified bodies are independent organizations designated by EU member states which are responsible for, among other things, auditing and examining a product’s technical dossiers and the manufacturers’ quality system. If satisfied that the relevant product conforms to the relevant essential requirements, the notified body issues a certificate of conformity, which allows the manufacturer to place the CE mark on the device and for it to be marketed throughout the EU. Given the additional requirements of the MDR, the renewal of our existing CE mark certificates once they expire or obtaining certifications for new products is more challenging, time consuming and costly.
For example, technical documentation for certain of our products requiring recertification, such as our single injection HA treatment Durolane®, and EXOGEN Bone Stimulation System have been submitted to our notified body. While we are actively engaged with our notified body to renew the CE marks for these and our other products, CE mark renewals for these products are still pending. Our inability to timely review and obtain CE mark certificates for these and other of our products could prohibit their distribution and marketing in EU member states, which would adversely affect our business, prospects, financial condition and results of operations.
Recent environmental regulatory actions regarding medical device sterilization facilities could result in disruptions in the supply us withof certain of our products whichand could adversely affect our business, results of operations and financial condition.
Our disposable products that are used with our neXus® Ultrasonic Surgical Aspirator System require sterilization using ethylene oxide prior to sale. Ethylene oxide sterilization is a common and scientifically proven sterilization method that is widely used in the medical device industry. We contract with third-party sterilizers to perform this service. Concerns about unsafe levels of ethylene oxide emissions in the air around some sterilization facilities have resulted in certain state environmental protection agency actions against those facilities that have impacted available capacity for medical device manufacturers’ to sterilize their devices. For example, recently the operations of certain of our contracted sterilization providers were temporarily suspended by the supplier as a voluntary response to a state environmental agency investigation. While such actions have not disrupted our ability to supply products and the previously shut down facilities have been permitted to resume certain operations after implementation of increased emissions controls, it is uncertain as to whether these facilities will be shut down or experience capacity reductions related to environmental, health and safety concerns. The U.S. Environmental Protection Agency has proposed new rules governing emissions from ethylene oxide sterilization facilities and has announced their intent to finalize the rule during 2024. It is unknown whether any other sterilization facilities we may contract with in the future will experience reduced capacity related to new regulatory requirements or will be required to shut down, either temporarily related to upgrading emissions controls or permanently due to inability to comply with new environmental regulation. To the extent that our third-party sterilizers are unable to sterilize our products, whether due to these regulatory or other limitations (such as capacity, reductions in operations, or availability of materials for sterilization), we may be unable to transition to other third-party sterilizers, sterilizer locations or sterilization methods in a timely or cost effective manner, or at all, which could have a material adverse impact on our results of operations and financial condition.
If our facilities are damaged or become inoperable, we will be unable to continue to research, develop and manufacture our products and, as a result, our business, results of operations and financial condition may be adversely affected until we are able to secure a new facility.
We do not have redundant manufacturing facilities. Our other facilities and equipment would be costly to replace and could require substantial lead-time to repair or replace. Our facilities may be harmed or rendered inoperable by natural disasters (including events caused by or intensified by climate change) or man-made disasters, including, but not limited to, tornadoes, flooding, fire and power outages. Such disasters may render it difficult or impossible to manufacture and commercialize our products and conduct our research and development activities for new products, line extensions and expanded indications. The inability to perform those activities, combined with our limited inventory of supplies, components and finished product, may result in the inability to continue manufacturing or supplying our products during such periods and the loss of customers or harm to our reputation. Although we possess insurance for damage to our facilities and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and this insurance may not continue to be available to us on acceptable terms, or at all.
Risks related to intellectual property matters
Protection of our intellectual property rights may be difficult and costly, and our inability to protect our intellectual property could adversely affect our competitive position.
Our success depends in part on our ability to protect our proprietary rights to the technologies and inventions used in, or embodied by, our products. To protect our proprietary technology, we rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, as well as nondisclosure, confidentiality and other contractual restrictions in our consulting and employment agreements. These legal means afford only limited protection, however, and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Our existing confidentiality and/or invention assignment agreements with employees, contractors, and others who participate in IP development activities could be breached, or we may not enter into sufficient and adequate agreements with those individuals in the first instance, and we may not have adequate remedies for such breaches. Furthermore, we may be subject to, and forced to defend against, third-party claims of ownership to our intellectual property. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or rights to use, valuable intellectual property. Such an outcome could adversely affect our business, results of operations and financial condition. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Patents
The process of applying for patent protection is time-consuming and expensive and we cannot assure you that all of our patent applications will issue as patents or that, if issued, they will issue in a form that will be advantageous to us. The rights granted to us under our patents including prospective rights sought in our pending patent applications, may not be meaningful or provide us with any commercial advantage, and they could be opposed, contested, narrowed, or circumvented by our competitors or declared invalid or unenforceable in judicial or administrative proceedings. We may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. As a result, some of our products are not, and in the future may not be, protected by patents. We generally apply for patents in those countries where we intend to make, have made, use, offer for sale, or sell products and where we assess the risk of infringement to justify the cost of seeking patent protection. However, we do not seek protection in all countries where we sell products and we may not accurately predict all the countries where patent protection would ultimately be desirable. If we fail to timely file a patent application in any such country or major market, we may be precluded from doing so at a later date. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories in which we have patent protection but where such protection may not be sufficient to terminate infringing activities. Furthermore, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed to us by third-parties.third parties. Therefore, these patents and applications may not be prosecuted or enforced in a manner consistent with the best interests of our business. If such licensors fail to maintain such patents, or lose rights to those patents, the rights we have licensed may be reduced or eliminated, which could also adversely affect our business, results of operations and financial condition.
We own numerous issued patents and pending patent applications relating to our technology and products. The rights granted to us under these patents, including prospective rights sought in our pending patent applications, could be opposed, contested or circumvented by our competitors or other third parties or declared invalid or unenforceable in judicial or administrative proceedings. If any of our patents are challenged, invalidated or legally circumvented by third-parties,third parties, and if we do not own other enforceable patents protecting our products, competitors could market products and use processes that are substantially similar to, or superior to, or otherwise competitive with those of ours, and our business willcould suffer. In addition, the patents we own or have licenses to may not be of sufficient scope or strength to provide us with any meaningful protection or commercial advantage, and competitors may be able to design around our patents or develop products that provide outcomes comparable to those of ours without infringing on our intellectualpatent property rights.
Even ifFurther, our patents are determined by the U.S. Patent and Trademark Office (USPTO) foreign patent office, or a court to be valid and enforceable, they may not be drafted or interpreted sufficiently broadly to prevent others from marketing products and services similar to ours or designing around our patents. For example, third parties may be able to develop products that are similar to ours but that are not covered by the claims of our patents. ThirdThird- parties may assert that we or our licensorsthe inventors of any patents licensed to us were not the first to make the inventions covered by our issued patents or pending patent applications. The claims of our issued patents or patent applications when issued may not cover our commercial technology or the future products and services that we develop. We may not have freedom to operate unimpeded by the patent rights of others. ThirdThird- parties may have dominating, blocking or other patents relevant to our technology of which we are not aware. In addition, because patent applications in the United States and many foreign jurisdictions are typically not published until 18eighteen months after the filing of certain priority documents (or, in some cases, are not published until they issue as patents) and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications foror published information which could invalidate our technologypatents or a portion of the claims of our contemplated technology.patents. Any such patent applications may have priority over our patent applications or issued patents, which could require us to obtain rights from third parties to issued patents or pending patent applications covering such technologies to allow us to commercialize our technology. If another party has filed a U.S. patent application on inventions similar to ours, depending on when the timing of the filing date falls under certain patent laws, we may have to participate in a priority contest (such as an interference proceeding) declared by the USPTO to determine priority of invention in the United States. There may be prior public disclosures of which we are not aware that could invalidate our patents or a portion of the claims of our patents. Further, we may not develop additional proprietary technologies and, even if we do, they may not be patentable.
In addition, patent reform legislation may pass in the future that could lead to additional uncertainties and increased costs surrounding the prosecution, enforcement, and defense of our patents and applications. We may be subject to a third-party preissuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or other patent office proceedings or litigation, in the United States or elsewhere, challenging our patent rights. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third-partiesthird parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
Moreover, the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In addition, periodic maintenance fees on issued patents often must be paid to the USPTO and foreign patent agencies over the lifetime of the patent. While an unintentional lapse can in manyIn some cases, be cured by payment of a late fee or by other means in accordancenoncompliance with the applicable rules, there are situations in which noncompliancesuch requirements can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or the owners of any patent rights licensed to us fail to maintain the patents and patent applications covering our products or procedures, we may not be able to stop a competitor from marketing products that are the same as or similar to our products, which would adversely affect our business, results of operations and financial condition.
Filing, prosecuting and defending patents on our products in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly developing countries, and the breadth of patent claims allowed can be inconsistent. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories in which we have patent protection that may not be sufficient to terminate infringing activities.
Due to differences between foreign and U.S. patent laws, our patented intellectual property rights may not receive the same degree of protection in every jurisdiction in which we obtain patents. Furthermore, we do not have patent rights in certain foreign countries in which a market may exist in the future. We may need to expend additional resources to protect or defend our intellectual property rights in these countries, and the inability to protect or defend the same could impair our brand or adversely affect the growth of our business internationally. For example, we may not be able to stop a competitor from marketing and selling in foreign countries products that are the same as, or similar to, or competitive with our products.
Patents have a limited lifespan, and the protection patents affords is limited. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Even if patents covering our products are obtained, once the patent life has expired for patents covering a product, we may be open to competition from competitive products and services. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing product candidates similar or identical to ours.
Trademarks
We rely on our trademarks as one means to distinguish our products from the products of our competitors, and have registered or applied to register many of these trademarks. However, we may not be able to successfully secure trademark registrations for all such applications.applications in each jurisdiction in which the product is marketed. Third-parties may oppose our trademark applications, or otherwise challenge our use of both registered and unregistered trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources to advertising and marketing new brands. Our competitors may infringe our trademarks and we may not have adequate resources to enforce our trademarks. Over the long term, if we are unable to establish name recognition based on our trademarks, then we may not be able to compete effectively and our business, results of operations and financial condition may be adversely affected.
Trade secrets and know-how
We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by consultants, vendors, former employees or current employees, despite the existence generally of confidentiality agreements and other contractual restrictions. Monitoring unauthorized uses and disclosures of our intellectual property is difficult, and we do not know whether the steps we have taken to protect our intellectual property will be effective.
Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. For example, the FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all. Our competitors could use any of the information we may be required to disclose by the FDA to develop independently technology similar to those of ours. Competitors could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property is not adequately protected so as to protect our market against competitors’ products and methods, our competitive position could be adversely affected, as could our business, results of operations and financial condition.
If we were to enforce a claim that a third-party had illegally obtained, misappropriated or was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. If any of the technology or information that we protect as trade secrets were to be independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may adversely affect our business, results of operations and financial condition. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret.
We depend on certain technologies that are licensed to us. We do not control the intellectual property rights covering these technologies and any loss of our rights to these technologies or the rights licensed to us could prevent us from selling our products, which could adversely impact our business, results of operations and financial condition.
We are a party to license agreements under which we are granted rights to intellectual property that is importantmaterial to our business, and we may need to enter into additional license agreements in the future. We rely on these licenses in order to be able to use and sell various proprietary technologies that are material to our business, as well as technologies which we intend to use in our future commercial activities. Our rights to use these technologies and the inventions claimed in the licensed patents are subject to the continuation of and our compliance with the terms of those licenses. Our existing license agreements impose, and we expect that future license agreements will impose on us, various diligence obligations, payment of milestones or royalties and other obligations. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, the licensor may have the right to terminate the license, in which case we would not be able to market products covered by the license, which would adversely affect our business, results of operations and financial condition.
As we have done previously, we may need to obtain licenses from third parties to advance our research or allow commercialization of our products and technologies. We may fail to obtain any of these licenses on commercially reasonable terms, if at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In the event that we are not able to acquire a license, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products and technologies, which could materially harm our business. In addition, the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties or other forms of compensation and damages.
In some cases, we may not have the right to control the prosecution, maintenance, or filing of the patents that are licensed to us, or the enforcement of these patents against infringement by third parties. Some of our patents and patent applications were not filed by us, but were either acquired by us or are licensed from third parties. Thus, these patents and patent applications were not drafted by us or our attorneys, and we did not control or have any input into the prosecution of these patents and patent applications prior to our acquisition of, or our entry into a license with respect to, such patents and patent applications. We cannot be certain that the drafting or prosecution of the patents and patent applications licensed to us will result or has resulted in valid and enforceable patents. Further, we do not always retain complete control over our ability to enforce our licensed patent rights against third-party infringement. In those cases, we cannot be certain that our licensor or other ultimate owner of such patents will elect to enforce these patents to the extent that we would choose to do so, or in a way that will ensure that we retain the rights we currently have under our license. If our licensor or other ultimate owners of such patents fails to properly enforce the patents subject to our license in the event of third-party infringement, our ability to retain our competitive advantage with respect to our products may be materially and adversely affected.
Licensing of intellectual property is an important part of our business and involves complex legal, business and scientific issues. Disputes may arise between us and our licensors regarding intellectual property that is subject to a license agreement, including:
•the scope of rights granted under the license agreement and other interpretation-related issues;
•whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the license agreement;
•our right to sublicense patent and other rights to third parties under collaborative development relationships;
•our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our products and technologies, and what activities satisfy those diligence obligations; and
•the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners.
In addition, we may become the owner of intellectual property that was obtained through assignments which may be subject to re-assignment back to the original assignor upon our failure to prosecute or maintain such intellectual property, upon our breach of the agreement pursuant to which such intellectual property was assigned, or upon our bankruptcy.
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, or if intellectual property is re-assigned back to the original assignor, we may be unable to successfully develop and commercialize the affected products and technologies.
Our intellectual property agreements with third parties may be subject to disagreements over contract interpretation, which could narrow the scope of our rights to the relevant intellectual property or technology.
Certain provisions in our intellectual property agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could affect the scope of our rights to the relevant intellectual property or technology, or affect financial or other obligations under the relevant agreement, either of which could adversely affect our business, results of operations and financial condition.
In addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact conceives or develops intellectual property that we regard as our own. Our assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
We may in the future be a party to patent and other intellectual property litigation and administrative proceedings that could be costly and could interfere with our ability to successfully market our products.
The medical device industry has been characterized by frequent and extensive intellectual property litigation and is highly competitive. Our competitors or other patent holders may assert that our products and/or the methods employed in our products are covered by their patents or that we are infringing, misappropriating, or misusing their trademark, copyright, trade secret, and/or other proprietary rights.
If our products or methods are found to infringe, we could be prevented from manufacturing or marketing our products. In the event that we become involved in such a dispute, we may incur significant costs and expenses and may need to devote resources to resolving any claims, which would reduce the cash we have available for operations and may be distracting to management and other employees, including those involved in the development of intellectual property. We do not know whether our competitors or potential competitors have applied for, will apply for, or will obtain patents that will prevent, limit or interfere with our ability to make, use, sell, import or export our products. Because patent applications can take many years to issue, third parties may have currently pending patent applications which may later result in issued patents that our products and technologies may infringe, or which such third parties claim are infringed by the use of our products or technologies. There is no guarantee that patents will not issue in the future from currently pending applications that may be infringed by our technology or products. In addition, identification of third-party patent rights that may be relevant to our technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases, and difficulty in assessing the meaning of patent claims. Moreover, as the medical device industry expands and more patents are issued in this area, the risk increases that we may be subject to claims of infringement of the patent rights of third parties. We cannot assure you that we will prevail in such actions, or that other actions alleging misappropriation or misuse by us of third-party trade secrets or infringement by us of third-party patents, copyrights, trademarks or other rights or challenging the validity of our patents, copyrights, trademarks or other rights will not be asserted against us. Competing products may also be sold in other countries in which our patent coverage might not exist or be as strong. If we lose a foreign patent lawsuit alleging our infringement
We may also initiate litigation against third-partiesthird parties to enforce our patent and proprietary rights or to determine the scope, enforceability or validity of the proprietary rights of others. Our intellectual property has not been tested in litigation. If we initiate litigation to protect our rights, we run the risk of having our patents and other proprietary rights invalidated, canceled or narrowed, which could undermine our competitive position. Further, if the scope of protection provided by our patents or patent applications or other proprietary rights is threatened or reduced as a result of litigation, it could discourage third parties from entering into collaborations with us that are important to the commercialization of our products.
We may be subject to ownership disputes relating to intellectual property, including disputes arising from conflicting obligations of consultants or others who are involved in developing our product. Furthermore, if a license to necessary technology is terminated, the licensor may initiate litigation claiming that our processes or products infringe or misappropriate its patent or other intellectual property rights and/or that we breached our obligations under the license agreement, and we and our collaborators would need to defend against such proceedings.
These lawsuits and proceedings, regardless of merit, are time-consuming and expensive to initiate, maintain, defend or settle, and could divert the time and attention of managerial and technical personnel, which could materially adversely affect our business, results of operations and financial condition. Any such claim could also force use to do one or more of the following:
•incur substantial monetary liability for infringement or other violations of intellectual property rights, which we may have to pay if a court decides that the product, service, or technology at issue infringes or violates the third-party’s rights, and if the court finds that the infringement was willful, we could be ordered to pay treble damages and the third-party’s attorneys’ fees;
•pay substantial damages to our customers or end users to discontinue use or replace infringing technology with non-infringing technology;
•stop manufacturing, offering for sale, selling, using, importing, exporting or licensing the product or technology incorporating the allegedly infringing technology or stop incorporating the allegedly infringing technology into such product, service, or technology;
•obtain from the owner of the infringed intellectual property right a license, which may require us to pay substantial upfront fees or royalties to sell or use the relevant technology and which may not be available on commercially reasonable terms, or at all;
•redesign our products, services, and technology so they do not infringe or violate the third-party’s intellectual property rights, which may not be possible or may require substantial monetary expenditures and time;
•enter into cross-licenses with our competitors, which could weaken our overall intellectual property position;
•lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property against others;
•find alternative suppliers for non-infringing products and technologies, which could be costly and create significant delay; or
•relinquish rights associated with one or more of our patent claims, if our claims are held invalid or otherwise unenforceable.
Some of our competitors may be able to sustain the costs of complex intellectual property litigation more effectively than we can because they have substantially greater resources.can. In addition, intellectual property litigation, regardless of its outcome, may cause negative publicity, adversely impact prospective customers, cause product shipment delays, divert the time, attention and resources of management, or prohibit us from manufacturing, marketing or otherwise commercializing our products, services and technology. Any uncertainties resulting from the initiation and continuation of any litigation could adversely affect our ability to raise additional funds or otherwise adversely affect our business, results of operations and financial condition.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If these results are perceived to be negative, the price of our Class A common stock could be adversely affected.
In addition, certain of our agreements with suppliers, distributors, customers and other entities with whom we do business may require us to defend or indemnify these parties to the extent they become involved in infringement claims relating to our technologies or products, or rights licensed to them by us. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify any of these third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, results of operation and financial condition.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or former employers or are in breach of non-competition or non-solicitation agreements with our competitors or former employers.
We could in the future be subject to claims that we or our employees have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of former employers or competitors. In addition, we may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and could be a distraction to management. If our defense to those claims fails, in addition to paying monetary damages, a court could prohibit us from using technologies or features that are essential to our products, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the competitors or former employers. An inability to incorporate technologies or features that are important or essential to our products could adversely affect our business, results of operations and financial condition, and may prevent us from selling our products. In addition, we may lose valuable intellectual property rights or personnel. Any litigation or the threat thereof may adversely affect our ability to hire employees or contract with independent sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our products, which could adversely affect our business, results of operations and financial condition.
Any product candidates that we develop as biologics subject to the BLA pathway may be subject to competition sooner than anticipated.
We expect to submit a BLA to allow for the marketing of MOTYS following the expiration of FDA’s enforcement discretion period for certain HCT/Ps. See “—Risks related to government regulation—Our HCT/P products are subject to extensive government regulation and our failure to comply with these requirements could cause our business to suffer. These products could be subject to significant additional regulatory requirements.” The Biologics Price Competition and Innovation Act of 2009 (BPCIA)(“BPCIA”) was enacted as part of the Affordable Care Act to establish an abbreviated pathway for the approval of biosimilar and interchangeable biological products. The regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an approved biologic. Under the BPCIA, an application for a biosimilar product cannot be approved by the FDA until 12 years after the reference product was approved under a BLA. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when processes intended to implement BPCIA may be fully adopted by the FDA, any of these processes could have a material adverse effect on the future commercial prospects for our biological products.
We believe that any of the product candidates we develop that is approved in the United States as a biological product under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider the subject product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of the reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
In addition, the approval of a biologic product biosimilar to one of our products could have a material adverse impact on our business as it may be significantly less costly to bring to market and may be priced significantly lower than our products.
Intellectual property rights do not necessarily address all potential threats to our business.
Once granted, patents may remain open to invalidity challenges including opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings for a given period after allowance or grant, during which time third parties can raise objections against such grant. In the course of such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims thus attacked, or may lose the allowed or granted claims altogether.
In addition, the degree of future protection afforded by our intellectual property rights is uncertain because even granted intellectual property rights have limitations, and may not adequately protect our business, provide a barrier to entry against our competitors or potential competitors or permit us to maintain our competitive advantage. Moreover, if a third-party has intellectual property rights that cover the practice of our technology, we may not be able to fully exercise or extract value from our intellectual property rights.
The following examples are illustrative:
•others may be able to develop and/or practice technology that is similar to our technology or aspects of our technology, but that are not covered by the claims of the patents that we own or control, assuming such patents have issued or do issue;
•we, or our licensorsthe inventors of any in-licensed patent rights, or any future strategic partners might not have been the first to conceive or reduce to practice the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed;
•we, or our licensorsthe investors of any in-licensed patent rights, or any future strategic partners might not have been the first to file patent applications covering certain of our inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
•our pending patent applications may not lead to issued patents;
•issued patents that we own or exclusively license may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges by our competitors;
•our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•third parties performing manufacturing or testing for us using our products or technologies could use the intellectual property of others without obtaining a proper license;
•parties may assert an ownership interest in our intellectual property and, if successful, such disputes may preclude us from exercising exclusive rights over that intellectual property;
•we may not develop or in-license additional proprietary technologies that are patentable;
•we may not be able to obtain and maintain necessary licenses on commercially reasonable terms, or at all; and
•the patents of others may adversely affect our business.
Should any of these events occur, they could adversely affect our business, results of operations and financial condition.
Risks related to our organizational structure and the Tax Receivable Agreement
Our principal asset is our interest in BV LLC, and, accordingly, we depend on distributions from BV LLC to pay our taxes and expenses, including payments under the Tax Receivable Agreement. BV LLC’s ability to make such distributions may be subject to various limitations and restrictions.
We are a holding company and have no material assets other than our ownership of LLC Interests of BV LLC. As such, we have no independent means of generating net sales or cash flow, and our ability to pay our taxes and operating expenses or declare and pay dividends in the future, if any, will be dependent upon the financial results and cash flows of BV LLC and its subsidiaries and distributions we receive from BV LLC. There can be no assurance that BV LLC and its subsidiaries will generate sufficient cash flow to distribute funds to us or that applicable state law and contractual restrictions, including negative covenants in our debt instruments, will permit such distributions.
BV LLC will continue to be treated as a partnership for U.S. federal income tax purposes and, as such, generally will not be subject to any entity-level U.S. federal income tax. Instead, taxable income will be allocated to holders of LLC Interests, including us. Accordingly, we will incur income taxes on our allocable share of any net taxable income of BV LLC. Under the terms of the Bioventus LLC Agreement, BV LLC will be obligated to make tax distributions to holders of LLC Interests, including us, subject to any limitations or restrictions in our debt arrangements. In addition to tax expenses, we will also incur expenses related to our operations, including payments under the Tax Receivable Agreement (TRA)(“TRA”), which we expect could be significant. See Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence-Tax Receivable Agreement in this Annual Reportfor further information. We intend, as its managing member, to cause BV LLC to make cash distributions to the owners of LLC Interests, including us, in an amount sufficient to (i) fund their or our tax obligations in respect of allocations of taxable income from BV LLC and (ii) cover our operating expenses, including payments under the TRA. However, BV LLC’s ability to make such distributions may be subject to various limitations and restrictions, such as restrictions on distributions that would either violate any contract or agreement to which BV LLC is then a party, including debt agreements, or any applicable law, or that would have the effect of rendering BV LLC insolvent. If we do not have sufficient funds to pay taxes or other liabilities or to fund our operations, we may have to borrow funds, which could materially adversely affect our liquidity and financial condition and subject us to various restrictions imposed by any such lenders. To the extent that we are unable to make payments under the TRA for any reason, such payments generally will be deferred and will accrue interest until paid; provided, however, that nonpayment for a specified period may constitute a material breach of a material obligation under the TRA and therefore accelerate payments due under the TRA. See Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Tax Receivable Agreement. In addition, if BV LLC does not have sufficient funds to make distributions, our ability to declare and pay cash dividends will also be restricted or impaired.
The TRA with the Continuing LLC Owner requires us to make cash payments to it in respect of certain tax benefits to which we are or may become entitled, and we expect that the payments we will be required to make could be significant.
We are a party to a TRA with the Smith & Nephew, Inc. (“Continuing LLC Owner.Owner”). Under the TRA, we are required to make cash payments to the Continuing LLC Owner equal to 85% of the tax benefits, if any, that we actually realize, or in certain circumstances are deemed to realize, as a result of (1) increases in the tax basis of assets of BV LLC resulting from (a) any future redemptions or exchanges of LLC Interests described under Part III, Item 13. Certain Relationships and Related Party Transactions, and Director of Independence—Bioventus LLC Agreement—LLC Interest Redemption Right, and (b) certain distributions (or deemed distributions) by BV LLC and (2) certain other tax benefits arising from payments under the TRA. We expect the amount of the cash payments that we will be required to make under the TRA will be significant. The actual amount and timing of any payments under the TRA will vary depending upon a number of factors, including the timing of redemptions or exchanges by the Continuing LLC Owner, the amount of gain recognized by the Continuing LLC Owner, the amount and timing of the taxable income we generate in the future, and the federal tax rates then applicable. Any payments made by us to the Continuing LLC Owner under the TRA will generally reduce the amount of overall cash flow that might have otherwise been available to us. To the extent that we are unable to make timely payments under the TRA for any reason, the unpaid amounts will be deferred and will accrue interest until paid by us. Furthermore, our obligation to make payments under the TRA could make us a less attractive target for an acquisition, particularly in the case of an acquirer that cannot use some or all of the tax benefits that are the subject of the TRA.
Payments under the TRA are not conditioned on the Continuing LLC Owner’s continued ownership of LLC Interests or our Class A common stock. For more information, see Part III, Item 13.Certain Relationships and Related Party Transactions, and Director of Independence—Tax Receivable Agreement. Assuming no material changes in the relevant tax laws and that we earn sufficient taxable income to realize all tax benefits that are subject to the TRA, we expect that the tax savings associated with future redemptions or exchanges of all remaining LLC Interests owned by the Continuing LLC Owner pursuant to the Bioventus LLC Agreement as described above, would aggregate to approximately $73.8 million over 20 years from the date of our IPO based on the IPO price of $13.00 per share of our Class A common stock and assuming all future redemptions or exchanges would occur by February 11, 2022. Under such scenario, assuming future payments are made on the date each relevant tax return is due, without extensions, we would be required to pay approximately 85% of such amount, or approximately $62.7 million, over the 20-year period from February 11, 2021. The actual amounts we will be required to pay under the TRA will depend on, among other things, the timing of subsequent redemptions or exchanges of LLC Interests by the Continuing LLC Owner, the price of our shares of Class A common stock at the time of each such redemption or exchange, and the amounts and timing of our future taxable income, and may be significantly different from the amounts described in the preceding sentence. Additionally, in certain cases such payments may be accelerated or significantly exceed the actual benefits we realize. See “—In certain cases, payments under the TRA to the Continuing LLC Owners may be accelerated or significantly exceed the actual benefits we realize in respect of tax attributes subject to the TRA.”
Our organizational structure, including the TRA, confers certain tax benefits upon the Continuing LLC Owner that may not benefit Class A common stockholders to the same extent as they will benefit the Continuing LLC Owner.
OurMoreover, our organizational structure, including the TRA, confers certain tax benefits upon the Continuing LLC Owner that may not benefit the holders of our Class A common stock to the same extent as they will benefit the Continuing LLC Owner. We entered intoRefer to risk factor—In certain cases, payments under the TRA with BV LLC and the Continuing LLC Owner that provides for our payment to the Continuing LLC Owner of 85% ofOwners may be accelerated or significantly exceed the amountactual benefits we realize in respect of tax benefits, if any, that we actually realize (or in some circumstances are deemedattributes subject to realize) as a result of (i) increases in the tax basis of assets of BV LLC resulting from (a) any future redemptions or exchanges of LLC Interests described under Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Bioventus LLC Agreement—LLC Interest Redemption Right, and (b) certain distributions (or deemed distributions) by BV LLC and (ii) certain other tax benefits arising from payments under the TRA. Although Bioventus will retain 15% of such tax benefits, this and other aspects of our organizational structure may adversely impact the future trading market for the Class A common stock.TRA.
In certain cases, payments under the TRA to the Continuing LLC Owner may be accelerated or significantly exceed the actual benefits we realize in respect of the tax attributes subject to the TRATRA.
The TRA provides that if (i) we materially breach any of our material obligations under the TRA, (ii) we undertake certain mergers, assetassets sales, other forms of business combinations or other changes of control were to occur on or before December 31, 2021 or (iii) we elect an early termination of the TRA, then our obligations or our successor’s obligations under the TRA to make payments thereunder would be based on certain assumptions, including an assumption that we would have sufficient taxable income to fully utilize all potential future tax benefits that are subject to the TRA (or, in the case of certain mergers, assetassets sales, other forms of business combinations or other changescharges of control, occurring after December 31, 2021, that we would have taxable income at least equal to four times the highest taxable income in any of the four fiscal quarters ending prior to the closing date of such transaction (increased by 10% for each taxable year beginning with the second taxable year following suchthe closing date)).
As a result of the foregoing, (i) we could be required to make payments under the TRA that are greater than the specified percentage of the actual benefits we ultimately realize in respect of the tax benefits that are subject to the TRA and (ii) if we materially breach any of our material obligations under the TRA or if we elected to terminate the TRA early, we would be required to make an immediate cash payment equal to the present value of the anticipated future tax benefits that are the subject of the TRA, which payment may be made significantly in advance of the actual realization, if any, of such future tax benefits.
In these situations, our obligations under the TRA could have a substantial negative impact on our liquidity and could have the effect of delaying, deferring or preventing certain mergers, asset sales, other forms of business combinations or other changes of control. For example, had we elected to terminate the TRA immediately following our IPO, assuming no material changes in the relevant tax laws or tax rates and that we earn sufficient taxable income to realize all tax potential benefits that are subject to the TRA, we estimate that the aggregate of termination payments would have been approximately $54.3 million based on our IPO price of $13.00 per share of our Class A common stock, and assuming LIBOR were to be 0.36%. There can be no assurance that we will be able to fund or finance our obligations under the TRA. We may elect to completely terminate the TRA early only with the written approval of a majority of our directors other than any directors that have been appointed or designated by the Continuing LLC Owner or any of such person’s affiliates.
We may make payments to the Continuing LLC Owner under the TRA that exceed the tax benefits actually realized by us in the event that any tax benefits are disallowed by a taxing authority.
Payments under the TRA are be based on the tax reporting positions that we determine, and the Internal Revenue Service (IRS)(“IRS”) or another tax authority may challenge all or part of the tax basis increases, as well as other related tax positions we take, and a court could sustain such challenge. Pursuant to the TRA, the Continuing LLC Owner is required to reimburse us for any cash payments previously made to it under the TRA in the event that any tax benefits actually realized by us and for which payment has been made under the TRA are subsequently challenged by a taxing authority and are ultimately disallowed. In addition, but without duplication of any amounts previously reimbursed by the Continuing LLC Owner, any excess cash payments made by us to the Continuing LLC Owner will be netted against any future cash payments that we might otherwise be required to make to the Continuing LLC Owner under the terms of the TRA. However, we might not determine that we have effectively made an excess cash payment to the Continuing LLC Owner for a number of years following the initial time of such payment. Moreover, there can be no assurance that any excess cash payments for which the Continuing LLC Owner has a reimbursement obligation under the TRA will be repaid to us. As a result, payments could be made under the TRA in excess of the tax savings that we realize in respect of the tax attributes with respect to the Continuing LLC Owner that are the subject of the TRA.
Unanticipated changes in effective tax rates or adverse outcomes resulting from examination of our income or other tax returns could adversely affect our results of operations and financial condition.
We are subject to taxes by the U.S. federal, state, local and foreign tax authorities, and our tax liabilities will be affected by the allocation of expenses to differing jurisdictions. Our future effective tax rates could be subject to volatility or adversely affected by a number of factors, including:
•changes in the valuation of our deferred tax assets and liabilities;
•expected timing and amount of the release of any tax valuation allowances;
•tax effects of equity-based compensation;
•changes in tax laws, regulations or interpretations thereof; or
•future earnings being lower than anticipated in countries where we have lower statutory tax rates and higher than anticipated earnings in countries where we have higher statutory tax rates.
factors. In addition, we may be subject to audits of our income, sales and other transaction taxes by U.S. federal, state, local and foreign taxing authorities. Outcomes from these audits could adversely affect our business, results of operations and financial condition.
If we were deemed to be an investment company under the Investment Company Act of 1940, as amended, or the 1940 Act, as a result of our ownership of BV LLC, applicable restrictions could make it impractical for us to continue our business as contemplated and could adversely affect our business, results of operations and financial condition.
Under Sections 3(a)(1)(A) and (C) of the 1940 Act, a company generally will be deemed to be an “investment company” for purposes of the 1940 Act if (i) it is, or holds itself out as being, engaged primarily, or proposes to engage primarily, in the business of investing, reinvesting or trading in securities or (ii) it engages, or proposes to engage, in the business of investing, reinvesting, owning, holding or trading in securities and it owns or proposes to acquire investment securities having a value exceeding 40% of the value of its total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis. We do not believe that we are an “investment company,” as such term is defined in either of those sections of the 1940 Act.
As the sole managing member of BV LLC, we control and operate BV LLC. On that basis, we believe that our interest in BV LLC is not an “investment security” as that term is used in the 1940 Act. However, if we were to cease participation in the management of BV LLC, our interest in BV LLC could be deemed an “investment security” for purposes of the 1940 Act.
We and BV LLC intend to conduct our operations so that we will not be deemed an investment company. However, if we were to be deemed an investment company, restrictions imposed by the 1940 Act, including limitations on our capital structure and our ability to transact with affiliates, could make it impractical for us to continue our business as contemplated and could adversely affect our business, results of operations and financial condition.
Bioventus is controlled by the Original LLC Owners, whose interests may differ from those of our public stockholders.
As of March 22, 2021,December 31, 2023, the Original LLC Owners control approximately 83.8%41.8% of the combined voting power of our common stock through their ownership of both Class A common stock and Class B common stock. The Original LLC Owners will, for the foreseeable future, have the ability to substantially influence us through their ownership position over corporate management and affairs, and will be able to control virtually all matters requiring stockholder approval. The Original LLC Owners are able to, subject to applicable law, and the voting arrangements, described in Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence, elect a majority of the members of our Board, control actions to be taken by us and our Board, including amendments to our certificate of incorporation and bylaws and approval of significant corporate transactions, including mergers and sales of substantially all of our assets. The directors so elected will have the authority, subject to the terms of our indebtedness and applicable rules and regulations, to issue additional stock, implement stock repurchase programs, declare dividends and make other decisions. It is possible that the interests of the Original LLC Owners may in some circumstances conflict with our interests and the interests of our other stockholders, including you. For example, the Continuing LLC Owner may have different tax positions from us, especially in light of the TRA that could influence our decisions regarding whether and when to dispose of assets, whether and when to incur new or refinance existing indebtedness, and whether and when Bioventus should terminate the TRA and accelerate its obligations thereunder. In addition, the determination of future tax reporting positions and the structuring of future transactions may take into consideration the Continuing LLC Owner’s tax or other considerations, which may differ from the considerations of us or our other stockholders.
Risks Relatedrelated to our ownership of our Class A common stock
In the past, we identified material weaknesses inOur stock price may be volatile or may decline regardless of our internal control over financial reporting. If we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, weoperating performance, and you may not be able to accurately or timely requirements applicable to public companies, which may adversely affect investor confidence in us, and, as a result, the market priceresell your shares of our Class A common stock.stock at or above the price at which you purchase them.
Ensuring that we have adequate internal financialThe stock market historically has experienced extreme price and accounting controls and procedures in place so that we can produce accurate financial statements onvolume fluctuations. As a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliabilityresult of financial reporting and the preparation of financial statements in accordance with GAAP.
A material weakness is a deficiency, or combination of deficiencies in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements willthis volatility, you might not be preventedable to sell your Class A common stock common stock at or detected on a timely basis.
In connection withabove the audit ofprice at which you purchase it. From our consolidated financial statements as of and forinitial public offering in February 2021 through February 27, 2024, the year ended December 31, 2020, we determined that we no longer have a material weakness associated with the proper processing of Exogen reimbursement claims in accordance with regulations and contractual terms. We implemented measures designed to improve our internal control over financial reporting to remediate such material weakness. These efforts included:
•the augmentation, reorganization and training of our prescription to cash staff, which includes our direct sales team, order management personnel, patient financial services personnel and reimbursement services and accounts receivable personnel, regarding key aspects of regulations and requirements and how to deal with inconsistencies within patient medical records;
•implementation of monthly sales order testing on sampling basis by our Compliance department including a review of medical necessity;
•establishment of a cross functional governance committee, reporting to an executive steering committee to review and approve our Exogen Medicare policy and oversee future Exogen policy and process interpretations and changes; and
•implementation of a checklist to be completed for each Medicare order to ensure compliance with our policy for Medicare claims and then further automating this checklist.
We cannot assure you that the measures we have taken to date, and actions we may take in the future, will be sufficient to prevent or avoid potential future material weaknesses. If we identify any additional material weaknesses, the accuracy and timing of our financial reporting may be adversely affected, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, investors may lose confidence in our financial reporting, and the marketper share trading price of our Class A common stock may declinehas been as a result. We could also become subjecthigh as $19.94 and as low as $0.80. It might continue to investigations byfluctuate significantly in response to various factors, some of which are beyond our control. These factors include:
•our operating performance and the SEC or other regulatory authorities, which could require additional financial and management resources.operating performance of similar companies;
Failure to establish and maintain effective internal controls in accordance with Section 404•the overall performance of the Sarbanes-Oxley Act could adversely affect our business and stock price.equity markets;
We are required to comply with the SEC’s rules implementing Sections 302 and 404 of the Sarbanes-Oxley Act, which require management to certify financial and other information•any major change in our quarterlymanagement;
•changes in laws or regulations relating to our products;
•announcements by us or our competitors of acquisitions, business plans, or commercial relationships;
•threatened or actual litigation;
•publication of research reports or news stories about us, our competitors, or our industry, or positive or negative recommendations;
•general political and annual reports and provide an annual management report on the effectivenesseconomic conditions.
Additionally, securities class action litigation has often been instituted against companies following periods of internal controls over financial reporting. Though we will be required to disclose changes made in our internal controls and procedures on a quarterly basis, we will not be required to make our first annual assessment of our internal control over financial reporting pursuant to Section 404 until the year following our first annual report required to be filed with the SEC. However, as an emerging growth company, our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 until the later of the year following our first annual report required to be filed with the SEC or the date we are no longer an emerging growth company. At such time, our independent registered public accounting firm may issue a report that is adversevolatility in the event it is not satisfied withoverall market and in the level at which our controls are documented, designed or operating.
To comply with the requirementsmarket price of being a public company, we have undertaken various actions, and may need to take additional actions, such as implementing new internal controls and procedures and hiring additional accounting or internal audit staff. Testing and maintaining internal controls cancompany’s securities. This litigation, if instituted against us, could result in substantial costs, divert our management’s attention from other matters that are important to the operation ofand resources, and harm our business. Additionally, when evaluating our internal controls overbusiness, operating results, and financial reporting, we may identify material weaknesses that we may not be able to remediate in time to meet the applicable deadline imposed upon us for compliance with the requirements of Section 404. If we identify any material weaknesses in our internal controls over financial reporting or are unable to comply with the requirements of Section 404 in a timely manner or assert that our internal controls over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal controls over financial reporting once we are no longer an emerging growth company, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our Class A common stock could be adversely affected, and we could become subject to investigations by the stock exchange on which our securities are listed, the SEC or other regulatory authorities, which could require additional financial and management resources.
We are a “controlled company” within the meaning of Nasdaq listing standards and, as a result, will qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections afforded to stockholders of companies that are subject to such requirements.
The Former LLC Owners and Continuing LLC Owner (Voting Group), which holds Class A common stock and Class B common stock representing approximately 83.8% of the combined voting power of our common stock, entered into a Stockholders Agreement (Stockholders Agreement) (see Part III, Item 13. Certain Relationships and Related Party Transactions, and Director of Independence). For a period of time, the parties to the Stockholders Agreement will agree to vote their shares of Class A common stock and Class B common stock in favor of the election of the nominees of certain members of the Voting Group to our Board upon their nomination by the nominating and corporate governance committee of our Board.
Because of the Stockholders Agreement and the aggregate voting power over our Class A common stock and Class B common stock held by the parties to the Stockholders Agreement, we are considered a “controlled company” for the purposes of Nasdaq. As such, we are exempt from certain corporate governance requirements of Nasdaq, including (1) the requirement that a majority of the Board consist of independent directors, (2) the requirement that we have a nominating and corporate governance committee that is composed entirely of independent directors and (3) the requirement that we have a compensation committee that is composed entirely of independent directors. We intend to rely on some or all of these exemptions. As a result, we do not have a majority of independent directors and our compensation and nominating and corporate governance committees do not consist entirely of independent directors. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
Taking advantage of the reduced disclosure requirements applicable to “emerging growth companies” may make our Class A common stock less attractive to investors.
The JOBS Act provides that, so long as a company qualifies as an “emerging growth company,” it will, among other things:
•be exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that its independent registered public accounting firm provide an attestation report on the effectiveness of its internal control over financial reporting;
•be exempt from the “say on pay” and “say on golden parachute” advisory vote requirements of the Dodd-Frank Wall Street Reform and Customer Protection Act, or the Dodd-Frank Act;
•be exempt from certain disclosure requirements of the Dodd-Frank Act relating to compensation of its executive officers and be permitted to omit the detailed compensation discussion and analysis from proxy statements and reports filed under the Exchange Act; and
•be permitted to provide a reduced level of disclosure concerning executive compensation and be exempt from any rules that have been adopted by the Public Company Accounting Oversight Board requiring a supplement to the auditor’s report on the financial statements or that may be adopted requiring mandatory audit firm rotations.
We are an “emerging growth company,” as defined in the JOBS Act, and we could be an emerging growth company for up to five years following our IPO. For as long as we continue to be an emerging growth company, we may choose to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies. We have irrevocably elected not to take advantage of the extension of time to comply with new or revised financial accounting standards available under Section 107(b) of the JOBS Act. We have also taken advantage of certain reduced reporting burdens in this Annual Report. We could be an emerging growth company for up to five years after our IPO and will continue to be an emerging growth company unless our total annual gross revenues are $1.07 billion or more, we have issued more than $1 billion in non-convertible debt in the past three years or we become a “large accelerated filer” as defined in the Exchange Act. If we remain an “emerging growth company”, we may take advantage of other exemptions, including the exemptions from the advisory vote requirements and executive compensation disclosures under the Dodd-Frank Act and the exemption from the provisions of Section 404(b) of the Sarbanes-Oxley Act. We cannot predict if investors will find our Class A common stock less attractive if we elect to rely on these exemptions, or if taking advantage of these exemptions would result in less active trading or more volatility in the price of our Class A common stock. Also, as a result of our intention to take advantage of some or all of the reduced regulatory and reporting requirements that will be available to us as long as we qualify as an “emerging growth company,” our financial statements may not be comparable to those of companies that fully comply with regulatory and reporting requirements upon the public company effective dates.
We do not currently expect to pay any cash dividends.
We do not anticipate declaring or paying any cash dividends to holders of our Class A common stock in the foreseeable future. We currently intend to retain future earnings, if any, to finance our growth. Any determination to pay cash dividends in the future will be at the sole discretion of our Board, subject to limitations under applicable law and may be discontinued at any time. In addition, our ability to pay cash dividends is currently restricted by the terms of our 2019 Credit Agreement. Therefore, you are not likely to receive any dividends on your Class A common stock for the foreseeable future, and the success of an investment in our Class A common stock will depend upon any future appreciation in its value. Consequently, investors may need to sell all or part of their holdings of our Class A common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment. There is no guarantee that our Class A common stock will appreciate in value or even maintain the price at which our stockholders have purchased our Class A common stock. Investors seeking cash dividends should not purchase our Class A common stock.
In addition, our operations are currently conducted entirely through BV LLC and its subsidiaries and our ability to generate cash to meet our debt service obligations or to make future dividend payments, if any, is highly dependent on the earnings and the receipt of funds from BV LLC and its subsidiaries via dividends or intercompany loans.condition.
Our amended and restated certificate of incorporation, to the extent permitted by applicable law, contains provisions renouncing our interest and expectation to participate in certain corporate opportunities identified or presented to certain of our Original LLC Owners.
Certain of the Original LLC Owners are in the business of making or advising on investments in companies and these Original LLC owners may hold, and may, from time to time in the future, acquire interests in or provide advice to businesses that directly or indirectly compete with certain portions of our business or the business of our suppliers. Our amended and restated certificate of incorporation provides that, to the fullest extent permitted by law, none of the Original LLC Owners or any director who is not employed by us or his or her affiliates will have any duty to refrain from engaging in a corporate opportunity in the same or similar lines of business as us. The Original LLC Owners may also pursue acquisitions that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. As a result, these arrangements could adversely affect our business, results of operations, financial condition or prospects if attractive business opportunities are allocated to any of the Original LLC Owners instead of to us.
We may issue shares of preferred stock in the future, which could make it difficult for another company to acquire us or could otherwise adversely affect holders of our Class A common stock, which could depress the price of our Class A common stock.
Our amended and restated certificate of incorporation will authorize us to issue one or more series of preferred stock. Our Board will have the authority to determine the preferences, limitations and relative rights of the shares of preferred stock and to fix the number of shares constituting any series and the designation of such series, without any further vote or action by our stockholders. Our preferred stock could be issued with voting, liquidation, dividend and other rights superior to the rights of our Class A common stock. The potential issuance of preferred stock may delay or prevent a change in control of us, discourage bids for our Class A common stock at a premium to the market price, and materially and adversely affect the market price and the voting and other rights of the holders of our Class A common stock.
Anti-takeoverCertain anti-takeover provisions in our governing documents and under Delaware law could make an acquisition of our companyCompany more difficult, limit attempts by our stockholders to replace or remove our current management, and depress the market price of our common stock.
OurCertain provisions of our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law contain provisions that could have the effect of renderingrender more difficult, delayingdelay or preventing an acquisition deemed undesirableprevent transactions that stockholders consider favorable, including transactions in which you might otherwise receive a premium for your shares of our common stock. These provisions might also prevent or frustrate attempts by our Boardstockholders to replace or remove management, and include the following provisions:provisions that:
•authorizingauthorize the issuance of “blank check” preferred stock that could be issued by our Board to increase the number of outstanding shares and thwart a takeover attempt;
•establishingestablish a classified Board so that not all members of our Board are elected at one time;
•provide the removal of directors only for cause;
•prohibitingprohibit the use of cumulative voting for the election of directors;
•limitinglimit the ability of stockholders to call special meetings or amend our bylaws;
•requiringrequire all stockholder actions to be taken at a meeting of our stockholders; and
•establishingestablish advance notice and duration of ownership requirements for nominations for election to the Board or for proposing matters that can be acted upon by stockholders at stockholder meetings.
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the DGCL,management, which prevents interested stockholders, such as certain stockholders holding more than 15% of our outstanding common stock from engagingcould in certain business combinations unless (i) prior to the time such stockholder became an interested stockholder, the board approved the transaction that resulted in such stockholder becoming an interested stockholder, (ii) upon consummation of the transaction that resulted in such stockholder becoming an interested stockholder, the interested stockholder owned 85% of the common stock or (iii) following board approval, the business combination receives the approval of the holders of at least two-thirds of our outstanding common stock not held by such interested stockholder. Because we have “opted out” of Section 203 of the DGCL in our amended and restated certificate of incorporation, the statute will not apply to business combinations involving us.
Any provision of our amended and restated certificate of incorporation, amended and restated bylaws or Delaware law that has the effect of delaying, preventing or deterring a change in control couldturn limit the opportunity for our stockholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware will be, to the fullest extent permitted by law, the sole and exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation provides that, unless we consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware, isor the Court of Chancery, will be, to the fullest extent permitted by law, the sole and exclusive forum for: (a) any derivative action, suit or proceeding brought on our behalf; (b) any action, suit or proceeding asserting a claim of breach of fiduciary duty owed by any of our directors, officers or stockholders to us or to our stockholders; (c) any action, suit or proceeding arising pursuant to any provision of the DGCL, our amended and restated certificate of incorporation or amended bylaws (as either may be amended from time to time); or, (d) any action, suit or proceeding asserting a claim governed by the internal affairs doctrine; provided that the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. The choice of forum provision in our amended and restated certificate of incorporation does not designate the Court of Chancery as the exclusive forum for any claim for which the applicable statute creates exclusive jurisdiction in another forum and, accordingly, does not apply to any claims brought to enforce any liability or duty created by the Exchange Act. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provision contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations and financial condition.
We are an emerging growth company and a smaller reporting company, and we cannot be certain if the reduced disclosure requirements applicable to us will make our Class A common stock less attractive to investors.
We are an “emerging growth company” pursuant to the provisions of the JOBS Act. For as long as we are an “emerging growth company,” we may take advantage of certain exemptions from reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including, for example, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, reduced disclosure obligations relating to the presentation of financial statements in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of our periodic reports, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding advisory “say-on-pay” votes on executive compensation and shareholder advisory votes on golden parachute compensation. We have availed ourselves of some of these reduced reporting obligations and exemptions in our SEC filings and expect to continue to do so in future SEC filings.
In addition, emerging growth companies can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we have chosen to “opt out” of such extended transition period, and as a result, we plan to comply with any new or revised accounting standards on the relevant dates on which non-emerging growth companies must adopt such standards. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
We will continue to qualify as an emerging growth company until the earliest of:
•The last day of our fiscal year following the fifth anniversary of the date of our IPO;
•The last day of our fiscal year in which have annual gross revenues of at least $1.235 billion or more;
•The date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt;
•The date on which we are deemed to be a “large accelerated filer,” as such terms is defined in the Exchange Act rules.
Even after we no longer qualify as an emerging growth company, we may still qualify as a smaller reporting company and rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations including regarding executive compensation.
We cannot predict if investors will find our Class A common stock less attractive because we may rely on the reduced disclosure requirements and exemptions applicable to emerging growth companies and/or smaller reporting companies. If some
investors find our Class A common stock less attractive as a result, there may be a less active trading market for our Class A common stock and our stock price may be more volatile
Item 1B.Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
Risk Management and Strategy
Bioventus maintains a cybersecurity risk management program that is designed to enable us to assess, identify, and manage risk associated with cybersecurity threats (the “Cybersecurity Program”). Our Cybersecurity Program is based on standards promulgated by the National Institute of Standards and Technology (“NIST”) and the United States Cybersecurity and Infrastructure Security Agency (“CISA”) and includes the following elements:
•Identification and assessment of cybersecurity threats based on periodic internal and external assessments and monitoring, information from internal stakeholders, and external publications and resources such as those made available by CISA.
•Technical and organizational safeguards designed to protect against identified threats, including documented policies and procedures, technical controls, and employee education and awareness.
•Processes designed to detect the occurrence of cybersecurity events and to respond to and recover from cybersecurity incidents.
•A third-party risk management process designed to manage cybersecurity risks associated with our service providers, suppliers, and vendors.
Our Cybersecurity Program is regularly evaluated by internal and external experts with the results of those reviews reported to senior management and the Audit and Risk Committee of the Board of Directors. We also actively engage with key vendors, industry participants and threat intelligence communities as part of our continuing efforts to evaluate and enhance the effectiveness of the Cybersecurity Program.
Integration of Risk Management Process
Assessing, identifying, and managing cybersecurity-related risks is integrated into our overall risk management framework. The Cybersecurity Program is integrated into our enterprise risk management program and framework. These programs are designed to foster a company-wide culture of appropriate cybersecurity risk management. Our IT Security team works closely with stakeholders across technology, legal, risk, and business operations to implement and monitor the effectiveness of the Cybersecurity Program.
Engagement of Third Parties in Connection with Risk Management
The Company engages a range of external experts to assist in its assessment, identification, and management of risks from cybersecurity threats. These include cybersecurity consultants and external auditors to review the Company’s cybersecurity posture and responsive efforts. Our relationships with these external partners enable us to leverage their expertise with the goal of maintaining best practices.
Oversight of Third-Party Risks
Our third-party service providers, suppliers, and vendors face their own risks from cybersecurity threats that could impact Bioventus in certain circumstances. We have implemented processes for overseeing and managing these risks. Those processes include assessing the third parties’ information security practices before allowing them to access our information systems or data, requiring the third parties to implement appropriate cybersecurity controls and otherwise agree to contractual requirements designed to address cybersecurity risks in our agreements with them, and conducting ongoing monitoring of their compliance with those requirements.
Risks from Cybersecurity Threats
As of the date of this Annual Report, we have not encountered any risks from cybersecurity threats that have materially affected or are reasonably likely to materially affect the Company, including its business strategy, results of operations, or financial condition. However, a third-party vendor recently informed us that Change Healthcare, a subsidiary of UnitedHealth Group that acts as an intermediary for processing certain of our claims for reimbursement related to our Exogen device to commercial payers experienced an incident in which a cybersecurity threat actor gained access to some of its information technology systems. As a result of the Change Healthcare incident, certain of our patient billing and collections processes have been disrupted. While we have identified an alternative claim processing intermediary and have resumed claims submissions to some payers, this event may cause delays in a portion of our claims submissions to some commercial payers thereby delaying the related cash remittances to us. As of the date of this Annual Report, UnitedHealth Group is still investigating this incident, including any potential impact on claims and patient data. On March 7, 2024, UnitedHealth Group issued a statement indicating that it expects to begin testing and reestablish connectivity to the effected claims network to restore service beginning March 18, 2024. We do not presently believe that the Change Healthcare incident has materially affected, or is reasonably likely to materially affect the Company, including with respect to our claims collection and cash flows. We continue to evaluate the impact of the Change Health incident on our Company.
Governance
The oversight of Bioventus’ Cybersecurity Program falls under the purview of the Company’s Director of IT Security, Risk and Compliance, who has over 25 years of combined technical and leadership experience, with the past 18 years focused on information security and technology risk management, and holds Certified Information Systems Security Professional (CISSP) and Certified Information Security Manager (CISM) certifications.
The Audit and Risk Committee of the Board of Directors is primarily responsible for the oversight of risks from cybersecurity threats, and is regularly briefed on the Company’s Cybersecurity Program by the Vice President of Information Technology and/or Director of IT Security, Risk and Compliance. These briefs include updates on the Company’s cyber risks and threats, the status of projects to strengthen our information security systems, assessments of the information security program, and the emerging cybersecurity threat landscape.
The Director of IT Security, Risk and Compliance implements and oversees our processes for regularly monitoring our information systems and detecting and reporting cybersecurity incidents. That process includes convening an incident response team composed of the Director of IT Security, Risk and Compliance, Vice President of Information Technology, Chief Compliance Officer, and General Counsel. The incident response team is responsible for overseeing the assessment of and response to any cybersecurity incident and for monitoring the Company’s mitigation and remediation efforts. The incident response team is also responsible for informing executive management, the Audit and Risk Committee and, where appropriate, the Board of Directors, regarding the detection, mitigation, and remediation of cybersecurity incidents.
Item 2.Properties.
Our principal executive offices are located on leased property in Durham, North Carolina. We also occupy leased office and manufacturing space in Cordova, Tennessee.Tennessee, Farmingdale, New York, and Valencia, California. In addition, our international operations occupy leased office spaces in Hoofddorp, Netherlands, Mississauga, Canada and Mississauga, Canada.Hod Hasharon, Israel. We believe that our facilities are sufficient to meet our current needs and that suitable additional space will be available as and when needed on acceptable terms.
Item 3.Legal Proceedings.
WeBioventus stockholder litigation
On January 12, 2023, the Company and certain of its current and former directors and officers were named as defendants in a putative class action lawsuit filed in the Middle District of North Carolina, Ciarciello v. Bioventus, Inc., No. 1:23– CV – 00032-CCE-JEP (M.D.N.C. 2023). The complaint asserts violations of Sections 10(b) and 20(a) of the Exchange Act and of Sections 11 and 15 of the Securities Act and generally alleges that the Company failed to disclose certain information regarding rebate practices, its business and financial prospects, and the sufficiency of internal controls regarding financial reporting. The complaint seeks damages in an unspecified amount. On April 12, 2023, the Court appointed Wayne County Employees’ Retirement System as lead plaintiff. The plaintiff’s amended consolidated complaint was filed with the Court on June 12, 2023. On July 17, 2023, the defendants filed a motion to dismiss the complaint raising a number of legal and factual deficiencies with the amended consolidated complaint. In response to the defendants’ motion to dismiss, the lead plaintiff filed a second amended complaint on July 31, 2023. The defendants moved to dismiss the second amended complaint on August 21, 2023, which the Court granted in part and denied in part on November 6, 2023. The Court dismissed the plaintiff’s Securities Act claims, but allowed the plaintiff’s Exchange Act claims to proceed into discovery.
On October 4, 2023, certain of the Company’s current and former directors and officers were named as defendants in a derivative shareholder lawsuit (in which the Company was a nominal defendant) filed in the United States District Court for the District of Delaware, Grogan, on behalf of Bioventus Inc., v. Reali, et.al., No. 1:23-CV-01099-RGA (D. Del. 2023). The complaint asserts violations of Section 14(a) of the Exchange Act, breaches of fiduciary duties and related state law claims, and a claim for contribution, and generally alleges the same purported misconduct as alleged in the Ciarciello case. On January 12, 2024, the Court agreed to stay this case pending resolution of the Ciarciello case.
On February 9, 2024, another plaintiff filed a derivative shareholder lawsuit against certain of the Company’s current and former directors and officers (in which the Company is a nominal defendant) filed in the United States District Court for the District of Delaware, Sanderson, on behalf of Bioventus Inc., v. Reali, et.al., No. 1:24-cv-00180-RGA (D. Del. 2024). Like the Grogan case, this case asserts violations of Section 10(b) of the Exchange Act, breaches of fiduciary duties and related state law claims, and a claim for contribution, and generally alleges the same purported misconduct as alleged in the Ciarciello case. The parties are in discussions to consolidate the two derivative matters and stay them on terms similar to those entered in the Grogan case.
The Company believes the claims alleged in each of the above matters lack merit and intends to defend itself vigorously. The outcome of the these matters is not currentlypresently determinable, and any loss is neither probable nor reasonably estimable.
Misonix former distributor litigation
On March 23, 2017, Misonix’s former distributor in China, Cicel (Beijing) Science & Technology Co., Ltd., filed a lawsuit against Misonix and certain of its officers and directors in the United States District Court for the Eastern District of New York. The complaint alleged that Misonix improperly terminated its contract with the former distributor. The complaint sought various remedies, including compensatory and punitive damages, specific performance and preliminary and post judgment injunctive relief, and asserted various causes of action, including breach of contract, unfair competition, tortious interference with contract, fraudulent inducement, and conversion. On October 7, 2017, the court granted Misonix’s motion to dismiss each of the tort claims asserted against Misonix, and also granted the individual defendants’ motion to dismiss all claims asserted against them. On January 23, 2020, the court granted Cicel’s motion to amend its complaint, to include claims for alleged defamation and theft of trade secrets in addition to the breach of contract claim. Discovery in the matter ended on August 5, 2021. On January 20, 2022, the court granted Misonix’s summary judgment motion on Cicel’s breach of contract and defamation claims. Cicel’s motion for reconsideration of the court’s summary judgment ruling in Misonix’s favor was dismissed by the Court on April 29, 2022. On July 18, 2022, Cicel voluntarily dismissed the remaining claim for trade secret theft and later filed an appeal to the United States Court of Appeals for the Second Circuit. On March 6, 2024, the Second Circuit Court of Appeals issued its ruling affirming the lower Court’s summary judgement in favor of Misonix in all respects.
Bioness stockholder litigation
On February 8, 2022, a minority shareholder of Bioness filed an action in the Delaware State Court of Chancery in connection with the Company’s acquisition of Bioness. Teuza, a Fairchild Technology Venture Ltd. v. Lindon, et. al., No. 2022-0130 -SG. This action names the former Bioness directors, the Alfred E. Mann Trust (Trust), which was the former majority shareholder of Bioness, the trustees of the Trust and Bioventus as defendants. The complaint alleges, among other things, that the individual directors, the Trust, and the trustees breached their fiduciary duty to the plaintiff in connection with their consideration and approval of the Company’s transaction. The complaint also alleges that the Company aided and abetted the other defendants in breaching their fiduciary duties to the plaintiff and that the Company breached the Merger Agreement by failing to pay the plaintiff its pro rata share of the merger consideration. The Company believes that it is indemnified under the indemnification provisions contained in the Bioness Merger Agreement for these claims. On July 20, 2022, the Company filed a motion to dismiss all claims made against it on various grounds, as did all the other named defendants in the suit. A hearing on Bioness’ and other the defendant’s motions was held before the Court of Chancery on January 19, 2023. On April 27, 2023, the Court issued an order which, among other things, dismissed Bioventus from the case.
Please refer to Part II, Item 8. Financial Statements and Supplementary Data—Notes to the Consolidated Financial Statements—Note 12. Commitments and contingencies of this Annual Report for additional information pertaining to legal proceedings. In addition, we are party to any material legal proceedings. We may at times be involved in litigationproceedings incidental to our business. While our management currently believes the ultimate outcome of these proceedings, individually and other legal claims in the ordinary courseaggregate, will not have a material adverse effect on our consolidated financial statements, litigation is subject to inherent uncertainties. Were an unfavorable ruling to occur, there exists the possibility of business. When appropriate in our estimation, we may record reserves ina material adverse impact on our financial statements for pending litigationcondition and other claims.results of operations.
Item 4.Mine Safety Disclosures.
Not applicable.Applicable.
PART II
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer
Purchases of Equity Securities.
Market Information and Holders
On February 11, 2021, we closed an initial public offering (IPO)(“IPO”) and our Class A common stock began trading on the Nasdaq Global Select Market under the symbol “BVS”.“BVS.” Prior to that time, there was no public market for our stock. There is no established public trading market for our Class B common stock.
Holders
As of March 22, 2021,February 27, 2024, we had 12approximately 167 holders of record of our Class A common stock. This amount does not take into account shareholders whose shares are held in “street name” by brokerage houses or other intermediaries. The closing price of our common stock on February 27, 2024 was $4.93. As of March 22, 2021,February 27, 2024, we had 1 holder of record of our Class B common stock.
Dividends
We do not anticipate declaring or paying any cash dividends to holders of our Class A common stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to finance the growth of our business. If we decide to pay cash dividends in the future, the declaration and payment of such dividends will be at the sole discretion of our board of directors (Board)(“Board”) and may be discontinued at any time. Holders of our Class B common stock are not entitled to participate in any dividends declared by our Board. In determining the amount of any future dividends, our Board will take into account any legal or contractual limitations, our actual and anticipated future earnings, cash flow, debt service and capital requirements and other factors that our Board may deem relevant.
In the event Bioventus Inc. declares any cash dividend, we intend to cause Bioventus LLC (BV LLC)(“BV LLC”) to make distributions to Bioventus Inc., in an amount sufficient to cover such cash dividends declared by us. If BV LLC makes such distributions to Bioventus Inc., the Class B common stock owner will also be entitled to receive the respective equivalent pro rata distributions in accordance with the percentages of their respective LLC Interests.
In addition, the terms of our financing arrangements contain covenants that may restrict BV LLC and its subsidiaries from paying such distributions, subject to certain exceptions. Any financing arrangements that we enter into in the future may include restrictive covenants that limit our ability to pay dividends. In addition, BV LLC is generally prohibited under Delaware law from making a distribution to a member to the extent that, at the time of the distribution, after giving effect to the distribution, liabilities of BV LLC (with certain exceptions) exceed the fair value of its assets. Subsidiaries of BV LLC are generally subject to similar legal limitations on their ability to make distributions to BV LLC.
UseEquity Compensation Plans
The information required by Item 5 of ProceedsForm 10-K regarding equity compensation plans is incorporated herein by reference to Part III, Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
On February 10, 2021, our Registration Statement on Form S-1 (File No. 333-252238) was declared effective byPerformance Graph
The following performance graph is not deemed to be “soliciting material” or to be “filed” with the SEC for our IPO pursuantor subject to whichRegulation 14A or 14C or to the liabilities of Section 18 of the Exchange Act. This information will not be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent we registered and sold an aggregate of 9,200,000 shares ofspecifically incorporate this information by reference.
The following performance graph compares the cumulative total return to stockholders on our Class A common stock (including 1,200,000 shares sold pursuantrelative to the underwriters' over-allotment option) at a price of $13.00 per share. Morgan Stanley & Co. LLC, J.P. Morgan Securities LLCcumulative total returns on the Nasdaq Composite Index and Goldman Sachs & Co. LLC acted as joint book-running managers in the offering. Canaccord Genuity Securities LLC acted as lead manager inS&P 500 Health Care Equipment Index for the offering. The offering commencedperiod commencing on February 11, 2021 and did not terminate before all of the securities registered in the registration statement were sold. The offering closed on February 16, 2021, resulting in net proceeds of $111.2 million after deducting underwriters' discounts and commissions of $8.4 million. No payments were made by us to directors, officers, general partners or persons owning 10% or more of(the date our common stock or to their associates, or to our affiliates.
We used the net proceeds to us from the IPO to purchase 9,200,000 newly-issued LLC Interests from BV LLC at a purchase price per interest equal to the IPO price per share of Class A common stock. As sole managing memberstock commenced trading on Nasdaq) through December 31, 2023 assuming an initial investment of BV LLC,$100. Nasdaq Composite Index and S&P 500 Health Care Equipment Index will not be deemed incorporate by reference into any other filings under the Exchange Act or the Securities Act, except to the extent we caused BV LLC to use the proceeds it received as follows: (i) to pay fees and expensesspecifically incorporate. Note that historic stock price performance is not necessarily indicative of approximately $3.8 million in connection with the IPO and the Transactions (ii) to satisfy the $3.3 million cash entitlementfuture stock price performance.
There has been no material change in the use of proceeds as described in the final prospectus filed on February 12, 2021.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2/11/21 | 3/31/21 | 6/30/21 | 9/30/21 | 12/31/21 | 3/31/22 | 6/30/22 | 9/30/22 | 12/31/22 | 3/31/23 | 6/30/23 | 9/30/23 | 12/31/23 |
| Bioventus Inc. | $ | 100.00 | | $ | 79.54 | | $ | 91.62 | | $ | 73.71 | | $ | 75.43 | | $ | 73.40 | | $ | 35.50 | | $ | 36.44 | | $ | 13.59 | | $ | 5.57 | | $ | 15.04 | | $ | 17.18 | | $ | 27.43 | |
| NASDAQ Composite | $ | 100.00 | | $ | 94.45 | | $ | 103.41 | | $ | 103.01 | | $ | 111.54 | | $ | 101.39 | | $ | 78.63 | | $ | 75.40 | | $ | 74.62 | | $ | 87.14 | | $ | 98.30 | | $ | 94.25 | | $ | 107.03 | |
| S&P 500 Health Care Equipment | $ | 100.00 | | $ | 96.27 | | $ | 104.57 | | $ | 109.55 | | $ | 113.11 | | $ | 106.11 | | $ | 85.61 | | $ | 79.47 | | $ | 90.75 | | $ | 93.13 | | $ | 102.06 | | $ | 88.00 | | $ | 97.83 | |
Item 6.[Reserved. [Reserved.]
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSManagement’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with Part I, Item 1A,1A. Risk Factors and our consolidated financial statements and the related notes to those statements included elsewhere in this Annual Report on Form 10-K (Annual Report)(“Annual Report”). In addition to historical consolidated financial information, the following discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Some of the numbers included herein have been rounded for the convenience of presentation. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed under Part 1, Item 1A. Risk Factors and elsewhere in this Annual Report. A discussion of the year ended December 31, 20192022 compared to the year ended December 31, 20182021 has been reported previously in our final prospectusForm 10-K for the fiscal year ended December 31, 2022, filed with the SEC on February 12, 2021,March 31, 2023, under the heading "Management's Discussion and Analysis of Financial Condition and Results of Operations."
Executive summarySummary
We are a global medical device company focused on developing and commercializing clinically differentiated, cost efficient and minimally invasive treatments that engage and enhance the body’s natural healing process. We operate our business through two reportable segments, U.S. and International, and our portfolio of products is grouped into three areas:
•Pain Treatments is comprised of non-surgical pain injection therapies as well as peripheral nerve stimulation (“PNS”) products to help the following three verticals:patient get back to their normal activities.
•OA joint pain treatment and joint preservation products, which are HA viscosupplementation therapies approved by the FDA through a PMA;
•BGSs, which are human tissue allograft and synthetic products used primarilySurgical Solutions is comprised of bone graft substitutes (“BGS”) that increase bone formation to stimulate bone healing in spine surgery which have either (i) received 510(k) clearance, which is a premarket submission made to the FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device, or (ii) are regulated solely as Section 361 HCT/Ps, which means they are human cells, tissues and cellular and tissue-based products that do not require a PMA in the United States; and
•minimally invasive fracture treatment, which is a FDA-approved Exogen system prescribed for long bone stimulation for fracture healing.
Our U.S. segment offers our full existing portfolio of products. This includes our OA joint pain treatment and joint preservation products, which address the entire market for HA viscosupplementation with offerings for single, three and five injection therapies: (i) Durolane, a single injection therapy, which we launched in the United States in the first half of 2018 and also market outside the United States in more than 30 countries; (ii) GELSYN-3, a three injection therapy, which we have marketed in the United States since the second half of 2016; and (iii) SUPARTZ FX, a five injection therapy, which we have marketed in the United States since May 2012. Our U.S. segment also offers our BGS products, which are targeted at improving bone fusion rates following spinal fusionfusions and other orthopedic surgeries. Thesesurgeries, as well as a portfolio of ultrasonic products include allograft-derivedused for precise bone graft with growth factors (OsteoAMP)cutting and sculpting, soft tissue management (i.e., a DBM (Exponent), cancellous bonetumor and liver resections) and tissue debridement, in different preparations (PureBone), bioactive synthetics (Signafuse and Interface),various surgeries, including minimally invasive applications.
•Restorative Therapies is comprised of a collagen ceramic matrix (OsteoMatrix) and two bone marrow isolation systems (CellXtract and Extractor). Further, our U.S. segment offers our Exogen system, which we believe offers significant advantages over electrical based long bone stimulation systems, including documented mechanism of action, shorter treatment times and superior nonunion heal rates.
Our International segment offers Durolane,system, as well as devices designed to help patients regain leg or single injection therapy, OsteoAMP, our allograft-derived bone graft with growth factors, and our Exogen system.hand function due to stroke, multiple sclerosis or other central nervous system disorders.
The following table sets forth total net sales, net (loss) income from continuing operations,and Adjusted EBITDA and pro forma net income (loss) per unit attributable to common unit holders - basic and diluted:for the periods presented:
| | | | | | | | | | | |
| Years Ended December 31, |
| 2020 | | 2019 |
| Net sales | $ | 321,161 | | | $ | 340,141 | |
| Net income from continuing operations | $ | 14,722 | | | $ | 8,113 | |
Adjusted EBITDA(1) | $ | 72,443 | | | $ | 79,188 | |
Net income (loss) per unit attributable to common unit holders - basic and diluted(2) | $ | 0.89 | | | $ | (0.13) | |
| | | | | | | | | | | | | | | |
| | | Years Ended December 31, |
| (in thousands, except for loss per share) | | | | | 2023 | | 2022 |
| Net sales | | | | | $ | 512,345 | | | $ | 512,117 | |
| Net loss from continuing operations | | | | | $ | (121,196) | | | $ | (144,651) | |
Adjusted EBITDA(1) | | | | | $ | 88,862 | | | $ | 68,610 | |
| Loss per Class A common stock, basic and diluted | | | | | | | |
| Continuing operations | | | | | $ | (1.54) | | | $ | (1.70) | |
| Discontinued operations | | | | | (0.95) | | | (0.89) | |
| Loss per Class A common stock, basic and diluted | | | | | $ | (2.49) | | | $ | (2.59) | |
(1)See below under Components Results of our results of operations-AdjustedOperations-Adjusted EBITDA for a definition of Adjusted EBITDA and Results from continuing operations for a reconciliation of net (loss) income from continuing operations to Adjusted EBITDA.
(2)See below for a computation of pro forma net income per unit attributable to common unit holders - basic and diluted after the recapitalization described below which was in conjunction with our IPO.
On February 16, 2021,May 22, 2023, we closed the sale of certain assets within our limited liability company agreement was amendedWound Business, including the TheraSkin and restatedTheraGenesis products (collectively, the “Wound Business” or the “Disposal Group”), for potential consideration of $84.7 million, including $34.7 million at closing, $5.0 million deferred for 18 months and up to among other things, (i) provide$45.0 million in potential earn-out payments (“Earn-out Payments”), which are based on the achievement of certain revenue thresholds by the purchaser of the Wound Business for a new single classsales of common membership intereststhe TheraSkin and TheraGenesis products during the 2024, 2025 and 2026 fiscal years.
We incurred $3.9 million in transactional fees resulting from the sale of the Wound Business. The deconsolidation of the Disposal Group resulted in the Company (LLC Interests)recognition of a $1.5 million loss on disposal of a business recorded within the consolidated statements of operations and (ii) exchange allcomprehensive (loss) income for the year ended December 31, 2023. The proceeds were used to prepay $30.0 million of long-term debt principal obligations.
We evaluated the existing membership interestsWound Business for LLC Interests. For purposesimpairment prior to its sale and recorded a $78.6 million impairment within the consolidated statements of calculating pro forma earnings per unit, we have adjusted the number of outstanding membership units retroactively to give effect to the amendmentoperations and resulting recapitalization.
Pro forma basic netcomprehensive (loss) income per unit is computed by dividing net income by the pro forma weighted-average number of units outstanding during the period. Pro forma diluted net income per unit is computed by dividing net income by the pro forma weighted-average number of units outstanding adjusted to give effect to potentially dilutive securities. Asyear ended December 31, 2023 as a result of this evaluation to reduce the recapitalization and the New LLC Owner assuming the obligationsintangible assets of the Company’s Phantom Plan awards there are no dilutive securities.
Disposal Group to reflect their respective fair values, less any costs to sell. The following table sets forth a reconciliationfair value of the numerators and denominators used to compute pro forma basic and diluted net income per unitDisposal Group’s intangibles were determined based on the consideration received for the years ended December 31 as follows:
| | | | | | | | | | | |
| 2020 | | 2019 |
Net income from continuing operations attributable to
common unit holders | $ | 16,411 | | | $ | 8,666 | |
| Loss from discontinued operations, net of tax | — | | | 1,815 | |
| Net income attributable to common unit holders | $ | 16,411 | | | $ | 6,851 | |
Pro forma net income per unit attributable to common unit holders
—basic and diluted | | | |
| Pro forma net income from continuing operations | $ | 0.29 | | | $ | 0.15 | |
| Pro forma loss from discontinued operations, net of tax | — | | | 0.03 | |
| Pro forma net income attributable to common unit holders | $ | 0.29 | | | $ | 0.12 | |
| | | |
Pro forma weighted average units used in computing basic and diluted
net income per common unit | 56,825,325 | | | 56,825,325 | |
Strategic transactions
We have pursued and continue to pursue business development opportunities that leverage our significant customer presence across orthopedics, broaden our portfolio and increase our global footprint. Below is a summary of some of our recent transactions:
Collaboration and development agreement for MOTYS
On May 29, 2019, we entered into a collaboration and development agreement (Development Agreement), with Musculoskeletal Transplant Foundation, Inc., or MTF, to develop an injectable placental tissue product, MOTYS, for use in the OA joint pain treatment. The development and commercialization of the product is anticipated to take place in two stages. In consideration for achieving its development milestones, we paid MTF $1.5 million and are obligated to pay additional payments totaling $0.8 million if certain further milestones are achieved. We began selling MOTYS in the fourth quarter of 2020, subject to the terms of an exclusive commercial supply agreement entered into with MTF on June 18, 2020.
Development collaboration agreement for PROcuff
On August 23, 2019, we entered into an exclusive Collaboration Agreement with Harbor, to develop and license the rights to commercialize a woven-suture-collagen composite implant product. Concurrently with the execution of the agreement, we purchased $1.0 million of shares of Harbor. As a result of Harbor’s achievement of certain milestones, on October 5, 2020, we purchased $1.0 million of additional shares of Harbor. Furthermore, we are obligated to make two additional one-time payments totaling $6.0 million in aggregate upon Harbor’s achievement of (i) receiving regulatory approval and (ii) achieving a certain net sales target. The sole use of proceeds from these investments is for the development of the implant product that is the subject of our agreement. We intend to negotiate and enter into a definitive supply agreement with Harbor if and when the product is cleared for marketing by the FDA at a price per unit not to exceed an agreed upon maximum.Wound Business.
CartiHeal (developer of Agili-C) investment and option and equity purchase agreement
On July 15, 2020,12, 2022, we madeacquired 100% of CartiHeal (2009) Ltd. (“CartiHeal”), a $15.0 million equity investment in CartiHeal, a privately-heldprivately held company headquartered in Israel and the developer of the proprietary Agili-C implant for the treatment of joint surface lesions in traumatic and osteoarthritic joints. This investment followsWe purchased CartiHeal (the “CartiHeal Acquisition”) for an aggregate purchase price of approximately $315.0 million and an additional $135.0 million, becoming payable after closing upon the recently completed enrollmentachievement of a certain sales milestone (“Sales Milestone Consideration”). We paid $100.0 million of the aggregate purchase price upon closing, consisting of a $50.0 million escrow deposit and outcome$50.0 million from a financing arrangement. We also paid approximately $8.6 million of interim analysis in CartiHeal’s IDE multinational pivotal study for Agili-C. This new roundtransaction-related fees and expenses and deferred $215.0 million (“Deferred Amount”) of funding is expected to enable CartiHeal to complete all patient follow-up in the Agili-C study and submit an application for PMAaggregate purchase price otherwise due at closing until the earlier of the achievement of certain milestones or the occurrence of certain installment payment dates. We recognized a gain of $23.7 million due to the FDA. Underchange in fair value of our equity method investment in CartiHeal as a result of the agreement, CartiHeal can secure an additional $5.0purchase during the third quarter of 2022. The gain was recognized in other income within the consolidated statement of operations and comprehensive (loss) income.
million equity investment from us, if needed, for IDE study completion. We previously made an initial $2.5 million investment in CartiHeal in January 2018 and a subsequent investment of $0.2 million in January 2020.
Contemporaneous with the July 2020 investment, we also entered into an Option and Equity Purchase Agreement with CartiHeal and its shareholders, which provides(“Option Agreement”) in July 2020. The Option Agreement provided us with an exclusive option to acquire 100% of CartiHeal’s shares under certain conditions, or the (“Call Option,Option”), and providesprovided CartiHeal with a put option that would require us to purchase 100% of CartiHeal’s shares under certain conditions, or the Put Option. The Call Option is exercisable by us at any time after the closing of the investment. The Put Option is only exercisable byconditions. In August 2021, CartiHeal uponachieved pivotal clinical trial success, including achievement of certain secondary endpointsas defined in the Option Agreement, for the Agili-C implant. In order to preserve our Call Option, in accordance with the Option Agreement and FDAupon approval of the Agili-C device with a label consistentBoard of Directors (“BOD”), we deposited $50.0 million into escrow in all respects with pivotal clinical trial success. The pivotal clinical trial’s objective isAugust 2021 for the potential acquisition of CartiHeal.
In April 2022, we exercised our Call Option to demonstrate the superiorityacquire all of the Agili-C implant over the surgical standardremaining shares of care, including microfracture and debridement, for the treatment of cartilage or osteochondral defects, in both osteoarthritic knees and knees without degenerative changes. If not previously exercised,CartiHeal, excluding shares we already owned. Our decision to exercise the Call Option andfollowed the Put Option terminate 45 days following the FDAFDA’s March 29, 2022 premarket approval of CartiHeal’s Agili-C orimplant. On June 17, 2022, the Company entered into an amendment to the Option Agreement with CartiHeal (“CartiHeal Amendment”) and Elron Ventures Limited, in its capacity as the event of failureshareholder representative, that provided for deferred payment of the pivotal clinical trial. We also haveconsideration for CartiHeal to be paid in multiple tranches, one of which was $50.0 million due upon the rightearliest to terminate the Call Option and Put Option at any time ending 30 days after receipt from CartiHealoccur of the statistical report regardingpublication in a peer-reviewed orthopedic journal of an article that presents the final results of the pivotal clinical trial upon(“First Paper Milestone”) or July 1, 2023.
Pursuant to the CartiHeal Amendment, we agreed to pay interest on each tranche of the Deferred Amount at a rate of 8.0% annually, until such tranche is paid.
The First Paper Milestone under the Option Agreement occurred on February 13, 2023, triggering our obligation to make the first $50.0 million payment, plus applicable interest, under the Option Agreement.
On February 27, 2023, we entered into a settlement agreement (the “Settlement Agreement”) with Elron Ventures Ltd. (“Elron” and together with the Company, the “Parties”) as representative of CartiHeal’s selling securityholders under the Option Agreement collectively, the “Former Securityholders”). Pursuant to the Settlement Agreement, Elron, on behalf of the Former Securityholders, agreed to forbear from initiating any legal action or proceedings relating to non-payment of any obligations arising under the Option Agreement during a period of 30 calendar days (the “Interim Period”) in exchange for (i) a one-time non-refundable amount of $10.0 million and (ii) a one-time non-refundable payment of a break fee of $30.0 million. Consideration for the acquisition of all of the shares of CartiHeal pursuant$0.2 million to the Call Option or Put Option would be $350.0 million, all of which would be payable at closing, with an additional $150.0 million payable upon achievement of certain sales milestones related to Agili-C. Such closing would be subject to customary closing conditions.
Certain of the foregoing transactions have had a significant impact on our financial results of operations for the periods in which they occurred, and they have affected the comparability of these statements for the corresponding comparative periods.
Outlook
We plan to continue to expand our business and to increase our net sales and profitability by executing on the following strategies:
•continue to expand market share in HA viscosupplementation;
•introduce new OA joint pain treatment and joint preservation products;
•further develop and commercialize our BGS portfolio;
•expand indications for use for our Exogen system;
•invest in research and development;
•pursue business development opportunities; and
•opportunistically grow our international markets.
We expect to face challenges as we execute on our business strategy. Our industry is highly competitive, subject to rapid change and significantly affected by market activities of industry participants, new product introductions and other technological advancements. We believe our experienced management team positions us for success in facing these and other challenges. However, there are several factors affecting our business that are beyond our control, such as our ability to successfully introduce new products and line extensions, expand labels, continue to obtain reimbursement for our products at acceptable rates and receive necessary governmental approvals. In addition, we expect that market demand, government regulation, third-party coverage and reimbursement policies and societal pressures will continue to change the healthcare industry worldwide, resulting in further business consolidations and alliances among our customers, which may exert further downward pressure on the prices of our products. For information about additional factors that may affect our outlook, see Part I, Item 1A. Risk Factors and “Special note regarding forward-looking statements” sections of this Annual Report.
COVID-19 Update and Outlook
During 2020, the COVID-19 pandemic spread around the world and in the U.S. and, more recently, new variants of the virus have emerged, some of which have shownElron to be more contagious. The COVID-19 pandemic has had widespread, rapidly evolving and unpredictable impacts on global society, economies, financial markets and business practices. Federal and state governments responded by implementing a number of measures designed to prevent or minimize its spread and the ongoing effects of the pandemic, including stay-at-home or shelter-in-place orders, quarantines and closure of all non-essential businesses, social distancing, travel restrictions, border closures, and other measures, which have caused global business disruptions and significant volatility in U.S. and international debt and equity markets.
The COVID-19 pandemic has rapidly escalated in the United States, creating significant uncertainty and economic disruption, and leading to record levels of unemployment nationally. Due to the evolving nature of the COVID-19 crisis, we continue to monitor the situation closely and assess the impact on our business. In response to various governmental orders and public health advisories, we have implemented a number of measures to protect the health and safety of our workforce, conserve liquidity and position us to emerge from the current crisis in a healthy financial position. These measures include closing our offices and instituting work-from-home policies with the exception of essential personnel in March 2020. In addition, we temporarily imposed employee salary reductions for our U.S. employees for the month of June 2020 and suspended, until December 31, 2020, a portion of the employer contribution we make under our 401(k) plan. All temporary salary reductions have now been reversed and all salaries have been reinstated to pre-COVID-19 levels. To the extent permitted andused in accordance with guidance from public health officials and government agencies, we have begun to reopen our locations and resume normal operations where appropriate. It is possible our operations will continue to be impacted in 2021, however, the magnitude and durationexpense fund provisions of the impact is impossibleOption Agreement. The Interim Period expired on March 29, 2023 and we did not exercise our right to predict due to:
•uncertainties regardingextend the durationInterim Period. In addition, the Parties mutually released any further claims under the Option Agreement and related transaction documents, including without limitation a release by the Former Securityholders of any rights to enforce the provisions of the COVID-19 pandemicOption Agreement or make further monetary claims against us and/or our respective affiliates and the length of time over which the disruptions caused by COVID-19 will continue;representatives.
•the impact of governmental orders and regulations that have been, and may in the future be, imposed in response to the pandemic;
•the impact of COVID-19 on our suppliers, manufacturers and other third parties on which we rely;
•the deterioration of economic conditions in the United States, as well as record high unemployment levels, which could have an adverse impact on discretionary consumer spending; and
•uncertainty regarding the potential for additional wavesUpon execution of the COVID-19 crisisSettlement Agreement, we transferred 100% of our shares in CartiHeal to occur.a trustee (the “Trustee”) for the benefit of the Former Securityholders. We had no ownership interest and no voting rights during the Interim Period. We have concluded that upon execution of the Settlement Agreement, the Company ceased to control CartiHeal for accounting purposes, and therefore, deconsolidated CartiHeal (the “Deconsolidation”, or “Disposal”) effective February 27, 2023. We treated the Disposal as a discontinued operation. The loss upon disposal totaled $60.6 million and was recorded within loss from discontinued operations, net.
The COVID-19 pandemic beganCredit and Guaranty Agreement
On July 11, 2022, we amended our Credit and Guaranty Agreement, dated as of December 6, 2019 (as previously amended on August 29, 2021 and October 29, 2021) in conjunction with the CartiHeal Acquisition to, have a material impact onamong other things, provide for an $80.0 million term loan facility (“Term Loan Facility”). On March 31, 2023, we further amended our business duringCredit and Guaranty Agreement to, among other things, modify certain financial covenant provisions, waive the noncompliance at December 31, 2022 and increase the applicable interest rate. We were in compliance as of December 31, 2023 with the financial covenants as stated within the Credit and Guaranty Agreement. On January 18, 2024, we further amended our Credit and Guaranty Agreement to modify certain financial covenants. Refer to Liquidity and Capital Resources—Credit Facilities for further information.
MOTYS Update
During the second quarter of 2020. Since March 2020, various governmental orders2022, prior to obtaining the results from our Phase 2 trial, we elected to discontinue the development of MOTYS to focus our resources on other priorities, including the integration of our 2021 and public health advisories, including “shelter-in-place” orders and quarantines, have reduced or prevented patient access to hospitals and physicians. As a result, the number of both elective and non-elective procedures have been reduced2022 acquisitions and our sales have decreased.
In addition,expanded R&D and product development portfolio we could be further impacted if we begin to see delaysinherited with these acquisitions. We incurred $1.0 million and $4.3 million in payments from customers, return to more stringent “shelter-in-place” orders or advisories, facility closures or other reasons related toexpenditures during the pandemic. Despite the COVID-19 pandemic, we had positive cash flows for the yearyears ended December 31, 2020, due2023 and 2022, respectively, related to a lack of significant delays in payments from customers, decreases in expenses correlating to a related decrease in sales, reduction in travel expenses and the institution of various cost cutting measures provided us with positive cash flow during the year ended December 31, 2020. However, the extent to which COVID-19 could materially impact our future liquidity is uncertain.MOTYS. No further costs are expected on MOTYS.
Consolidated Appropriations Act
In April 2020, we received $1.2 millionJuly 2022, in funds from the HHS as part of the CARES Act Provider Relief Fund. We determined we have compliedconnection with the CARESConsolidated Appropriations Act, Provider Relief Fund conditions so that we may use2021 (“CAA”), the fundsCenters for Medicare and Medicaid Services (“CMS”) began utilizing new pricing information the Company reported to reimburse for health care related expenses and lost revenues attributableit pursuant to the public health emergency resulting from COVID-19. An additional $2.9 million was received fromnewly adopted reporting obligations to adjust the CARES Act Provider Relief Fund in July 2020. We have recognized these payments as other income withinMedicare payment to healthcare providers using our consolidated statementDurolane and Gelsyn-3 products.
Under the CARES Act, we have also taken advantage of the deferral of employer social security payroll tax payments. In April 2020, we began deferring all employer social security payroll tax payments for the remainder of the 2020 calendar year, with 50% of the taxes is deferred until December 31, 2021 and the remaining 50% deferred until December 31, 2022.
We are continuing to evaluate other aspects of the CARES Act, including the use of the employee retention tax credit. The employee retention tax credit provides an additional tax credit to employers that (i) have either fully or partially suspended operations because of government orders associated with COVID-19 or (ii) experience a substantial decline in income but continue to pay employees their wages.
Components of our results of operations
Net sales
We generate net sales from a portfolio of active healing products that serve physicians spanning the orthopedic continuum, including sports medicine, total joint reconstruction, hand and upper extremities, foot and ankle, podiatric surgery, trauma, spine and neurosurgery. We report sales net of contractual allowances, rebates and returns.
We sell our OA joint pain treatment and joint preservation products and minimally invasive fracture treatmentprimarily through our direct sales team, who manage and maintain the sales relationship with healthcare providers, distribution centers or specialty pharmacies. Certain surgical products are sold through independent distributors to hospitals so our neurosurgeon and orthopedic spine surgeon customers can use them in procedures. In certain international markets, we also sell to independent distributors on prearranged business terms, who manage or maintain the sales relationship with their physician customers. Refer to Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note 2. Summary of significantSignificant accounting policies to our Notes to consolidated financial statements for further information information.
We generally recognize revenue at the point in time when control is transferred to the customer, typically, infor example, when the case of our OA joint pain treatment and joint preservation products, when these products areproduct is shipped to the customer, and, in the case of our Exogen system, when the patient has accepted the product.
Our BGSs are primarily sold in the U.S. market through independent distributors. We generally consign our BGS products to hospitals so our neurosurgeon and orthopedic spine surgeon customers can use them in procedures. We recognize revenue basedproduct or upon consumption in a surgical procedure.
Cost of sales
Our cost of sales primarily consistconsists of costs of products purchased from our third-party suppliers, direct labor and allocated overhead associated with themanufacturing and assembly, of our Exogen system, excess and obsolete inventory charges, shipping, inspection and related costs incurred in making our products available for sale or use. In addition, cost of sales includes depreciation related to production as well as amortization of product-related intellectual property and distribution rights associated with commercialized products. Our OA joint pain treatment and joint preservation products and BGSCertain products are manufactured by or obtained from third-party suppliers primarily located in Japan, Switzerland, Sweden and the United States. We receive the components for our Exogen system from suppliers and assemble each system in-house at our Cordova, Tennessee facility. In the future, we expect our cost of sales to increase due to increased sales volume.
Gross profit and gross margin
Gross profit consists of net sales less cost of sales. We calculate gross margin as gross profit divided by net sales. Our gross margin has been and will continue to be affected by a variety of factors, including costs of products purchased from our third-party suppliers, manufacturing costs, product mix and implementation over time of cost-reduction strategies. We expect net sales and product mix to vary quarter by quarter and therefore our gross profit will likely fluctuate from quarter to quarter.
Selling, general and administrative expense
Selling, general and administrative expense primarily consists of salaries, benefits and other related costs, including equity-based compensation, for personnel employed in sales, marketing, finance, legal, compliance, administrative, information technology, medical education and training, quality and human resource departments. Selling, general and administrative expense also includes third-party marketing, supply chain and distribution, product recall costs, information technology, legal, human resources, insurance and facilities expenses, selling, general and administrative expenses also include commissions, generally based on a percentage of sales, to our direct sales team and independent distributors. We expect our selling, general and administrative expenses will increase with the continued expansion of our sales organization and commercialization of our current and pipeline products. We plan to hire more personnel to support the growth of our business. In addition, as a public company, we will be implementing additional procedures and processes to address the standards and requirements applicable to public companies. We expect to incur additional annual selling, general and administrative expenses related to these additional procedures and processes including, among other things, equity-based compensation, increased liability insurance for our directors and officers, director fees, reporting requirements of the SEC, transfer agent fees, hiring additional accounting, legal and administrative personnel, increased auditing and legal fees and similar expenses. We also expect a change in the timing over which compensation expense is recognized as a result of the termination of the Phantom Plan and the receipt by participants of shares of Class A common stock upon settlement of their awards, which settlement is expected to take place twelve months after the date of such termination. However, over time, as we grow our net sales, we expect selling, general and administrative expenses to decline as a percentage of net sales.
Research and development expense
Research and development expense primarily consists of employee compensation, equity compensation and related expenses, as well as contract research organization service expenses related to clinical trials. We expense internal research and development costs as incurred and research and development costs incurred by third parties as they perform contracted work. Our research and development expenses may vary substantially from period to period based on the timing of research and development activities. We are focused on internal research and development to broaden our product portfolio across all verticals, expand our Exogen system product labelproducts and undertake clinical research to support commercialization of all of our products.their commercialization. As a result, we expect our research and development expenses to increasevary from low to the mid-single digits as a percentage of net sales as we introduce new products, extend existing product lines and expand indications. We see significant opportunity to develop innovative and clinically differentiated products in-house with our experienced research and development team. We are currently funding our B.O.N.E.S. clinical study, which began enrollment in 2018 and is aimed at broadening the label of our Exogen system to include a broader range of bones that may be treated as fresh fractures in predisposed patients at risk of nonunion. In addition, we are planning preclinical and animal model studies for MOTYS and PROcuff. Clinical and preclinical development timelines, the probability of success and development costs can differ materially from expectations.
Restructuring costs
We have restructured portions of our operations and future restructuring activities are possible. Identifying and calculating the cost to exit operations requires certain assumptions to be made, the most significant of which are anticipated future liabilities. Although our estimates have been reasonably accurate in the past, significant judgment is required, and these estimates and assumptions may change as additional information becomes available and facts or circumstances change. Restructuring costs are recorded at estimated fair value. Key assumptions in determining the restructuring costs include negotiated terms and payments to terminate contractual obligations.
Restructuring costs primarily consist of employee severance, legal, consulting and temporary labor expenses. DuringRestructuring costs recorded in 2023 and 2022 are the periods presented, restructuring costs were associated withresult of: i) aligning our organizational and management cost structure to improve profitability and cash flow; and ii) headcount reductions related to 2021 acquisitions. Restructuring costs recorded in our international business to improve operating efficiency. Key assumptions in determining2021 were the restructuring costs includeresult of headcount reductions as well as terms and negotiated paymentsrelated to terminate certain contractual obligations.acquisitions.
Depreciation and amortization
Depreciation expense primarily consists of depreciation of computer equipment and software as well as demonstration and consignment inventory, leasehold improvements, furniture, fixtures, machinery and equipment. Amortization expense primarily consists of amortization expense related to customer relationships and other intangible assets.
Interest expense
Interest expense primarily consists of interest on our indebtedness, which currently consists of our term loan and revolving credit facility, which was incurred pursuant to the Amended 2019 Credit Agreement. We have previously entered into interest rate swaps to limit our exposure to changes in the variable interest rate on our term loan. Interest expense includes any fair value gain or losses on these swaps. Interest expense also includes the revaluation for the liability related to our Equity Participation Right, or EPR, Unit. The EPR Unit’s entitlement is 0.55% of available distributions arising from a distribution event as defined in the Bioventus LLC Agreement and was settled in cash as part of our IPO.
Other expense (income) expense
Other expense (income) expense primarily consists of foreign currency transaction and remeasurement gains and losses on transactions denominated in currencies other than our functional currency. Our foreign currency transaction and remeasurement gains and losses are primarily related to foreign currency denominated cash, liabilities and intercompany receivables and payables. Other expense (income) expense may also include certain nonrecurring items.
Income tax expense
The Company’s subsidiary, Bioventus LLC (BV LLC)(“BV LLC”), is a partnership for U.S. federal tax purposes. Accordingly, the members include the profits and losses of BV LLC in their income tax returns. Certain wholly-owned subsidiaries of BV LLC are taxable entities for U.S. or foreign tax purposes and file tax returns in their local jurisdictions. Income tax expense includes U.S. federal and state and international income taxes, including certain taxes applicable to BV LLC and U.S. federal income taxes for one of our subsidiaries that is treated as a corporation for U.S. federal tax purposes. Certain income and expense items in income tax returns are not reported in the same year as financial statements. We report the income tax effects of these differences as deferred income taxes. Valuation allowances recognized reduce the related deferred tax assets to an amount which will, more likely than not, be realized. We recognize interest and penalties related to unrecognized tax benefits as a component of income tax expense.
Bioventus Inc. is subject to U.S. federal, state and local income taxes at the prevailing corporate tax rates with respect to our taxable income. In addition to tax expenses, we are obligated to make payments under the TRA, which could be significant. The TRA obligates us to pay to the Continuing LLC Owner 85% of the amount of any realized tax benefits (or in some circumstances are deemed to realize) resulting from (i) increases in the tax basis of assets of BV LLC as a result of (a) any future redemptions or exchanges of LLC Interests described under Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Bioventus LLC Agreement—LLC Interest Redemption Right, and (b) certain distributions (or deemed distributions) by BV LLC and (ii) certain other tax benefits arising from our making payments under the TRA. For more information, see Part III, Item 13.8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note 11. Income taxes for additional information.
Income tax expense includes U.S. federal, state and international income taxes, including certain taxes applicable to BV LLC. Certain Relationshipsincome and Related Transactions,expense items in income tax returns are not reported in the same year as financial statements. We report the income tax effects of these differences as deferred income taxes. Valuation allowances recognized reduce the related deferred tax assets to an amount which will, more likely than not, be realized. We recognize interest and Director Independence—Tax Receivable Agreement.penalties related to unrecognized tax benefits as a component of income tax expense.
Non-GAAP Financial Measures - Adjusted EBITDA
We present Adjusted EBITDA, a non-GAAP financial measure, because we believe it is a useful indicator of our operating performance. Ourthat management uses Adjusted EBITDA principally as a measure of our operating performance as well as for planning purposes, including the preparation of our annual operating budget and believesfinancial projections. We believe that Adjusted EBITDA is useful to our investors because it is frequently used by securities analysts, investors and other interested parties frequently use it in their evaluation of the operating performance of companies in industries similar to ours. Our management also uses Adjusted EBITDA for planning purposes, including the preparation of our annual operating budget and financial projections. We define Adjusted EBITDA as net (loss) income (loss) from continuing operations before depreciation and amortization, provision of income taxes and interest expense, net, adjusted for the impact of certain cash, non-cash and other items that we do not consider in our evaluation of ongoing operating performance. These items include acquisition and related costs, impairment of goodwill, impairment of assets, restructuring and succession charges, equity compensation change in fair value of contingent consideration, COVID-19 benefits, succession and transition charges,expense, financial restructuring costs, loss on impairmentdisposal of intangible assets, losses associated with debt refinancing, restructuring costs, foreign currency impacta business and other non-recurring costs.items. Adjusted EBITDA by segment is comprised of net sales and costs directly attributable to a segment, as well as an allocation of corporate overhead costs. The allocation of corporate overhead costs is determined based on various methods but is primarily based on a ratio of net sales by segment to total consolidated net sales. See table within Results from continuing operations
Non-GAAP financial measures have limitations as an analytical tool and should not be considered in isolation or as a substitute for, or superior to, the financial information prepared and presented in accordance with U.S. GAAP. These measures might exclude certain normal recurring expenses. Therefore, these measures might not provide a complete understanding of the Company's performance and should be reviewed in conjunction with the U.S. GAAP financial measures. Additionally, other companies might define their non-GAAP financial measures differently than we do. Investors are encouraged to review the reconciliation of net income from continuing operationsthe non-GAAP measure provided in this Annual Report, including in all tables referencing Adjusted EBITDA, to Adjusted EBITDA.its most directly comparable U.S. GAAP measure.
Interim Periods
The Company reports quarterly interim periods on a 13-week basis within a standard calendar year. Each annual reporting period begins on January 1 and ends on December 31. Each quarter ends on the Saturday closest to calendar quarter-end, with the exception of the fourth quarter, which ends on December 31. The 13-week quarterly periods ended on March 28, June 27 and September 26 for the year ended December 31, 2020. As a result, the fourth and first quarters may vary in length depending on the calendar year.
Results of Continuing Operations
The following table sets forth components of our condensed consolidated statements of operations from continuing operations as a percentage of net sales for the periods presented:
| | Years Ended December 31, |
| 2020 | | 2019 |
| Years Ended December 31, | | | Years Ended December 31, |
| 2023 | | | 2023 | | 2022 |
| Net sales | Net sales | 100.0 | % | | 100.0 | % | Net sales | 100.0 | % | | 100.0 | % |
| Cost of sales (including depreciation and amortization) | Cost of sales (including depreciation and amortization) | 27.3 | % | | 26.7 | % | Cost of sales (including depreciation and amortization) | 35.9 | % | | 35.4 | % |
| Gross profit | Gross profit | 72.7 | % | | 73.3 | % | Gross profit | 64.1 | % | | 64.6 | % |
| Selling, general and administrative expense | Selling, general and administrative expense | 60.1 | % | | 58.3 | % | Selling, general and administrative expense | 59.4 | % | | 64.9 | % |
| Research and development expense | Research and development expense | 3.5 | % | | 3.3 | % | Research and development expense | 2.6 | % | | 4.7 | % |
| Restructuring costs | Restructuring costs | 0.2 | % | | 0.2 | % | Restructuring costs | 0.2 | % | | 1.3 | % |
| Change in fair value of contingent consideration | | Change in fair value of contingent consideration | 0.1 | % | | 0.2 | % |
| Depreciation and amortization | Depreciation and amortization | 2.3 | % | | 2.3 | % | Depreciation and amortization | 1.7 | % | | 1.9 | % |
| Operating income | 6.6 | % | | 9.2 | % |
| Impairment of assets | | Impairment of assets | 15.4 | % | | — | % |
| Impairment of goodwill | | Impairment of goodwill | — | % | | 24.3 | % |
| Loss on disposals | | Loss on disposals | 0.7 | % | | — | % |
| Operating loss | | Operating loss | (16.0 | %) | | (32.7 | %) |
The following table presents a reconciliation of net incomeloss from continuing operations to Adjusted EBITDA for the periods presented:
| | | | | | | | | | | | | | |
| | Years Ended December 31, |
| (in thousands) | | 2020 | | 2019 |
| Net income from continuing operations | | $ | 14,722 | | | $ | 8,113 | |
Depreciation and amortization(a) | | 28,643 | | | 30,316 | |
| Income tax expense | | 1,192 | | | 1,576 | |
| Interest expense | | 9,751 | | | 21,579 | |
Equity compensation(b) | | 10,103 | | | 10,844 | |
COVID-19 benefits, net(c) | | (4,123) | | | — | |
Succession and transition charges(d) | | 5,609 | | | — | |
Restructuring costs(e) | | 563 | | | 575 | |
Foreign currency impact(f) | | (117) | | | 8 | |
Equity loss in unconsolidated investments(g) | | 467 | | | — | |
Other non-recurring costs(h) | | 5,633 | | | 6,177 | |
| Adjusted EBITDA | | $ | 72,443 | | | $ | 79,188 | |
| | | | | | | | | | | | | | | |
| | | Years Ended December 31, |
| (in thousands) | | | | | 2023 | | 2022 |
| Net loss from continuing operations | | | | | $ | (121,196) | | | $ | (144,651) | |
| Interest expense, net | | | | | 40,676 | | | 12,021 | |
| Income tax expense (benefit), net | | | | | 85 | | | (44,374) | |
Depreciation and amortization(a) | | | | | 57,365 | | | 55,398 | |
Acquisition and related costs(b) | | | | | 5,694 | | | 21,731 | |
Restructuring and succession charges(c) | | | | | 2,331 | | | 7,453 | |
Equity compensation(d) | | | | | 2,722 | | | 17,585 | |
Financial restructuring costs(e) | | | | | 7,291 | | | — | |
Impairment of assets(f) | | | | | 78,615 | | | 10,285 | |
Impairment of goodwill(g) | | | | | — | | | 124,697 | |
Loss on disposal of a business(h) | | | | | 1,539 | | | — | |
Other items(i) | | | | | 13,740 | | | 8,465 | |
| Adjusted EBITDA | | | | | $ | 88,862 | | | $ | 68,610 | |
(a)Includes for the years ended December 31, 20202023 and 20192022, respectively, depreciation and amortization of $21.2 million$48,503 and $22.4 million$45,622 in cost of sales and also includes $7.4 million$8,862 and $7.9 million, respectively,$9,776 in operating expenses presented in the consolidated statements of operations and comprehensive income (loss), with the balance in research income.
(b)Includes acquisition and development.
(b)Represents compensation as well as the change in fair market value resulting from two equity-based compensation plans, the MIP and the Phantom Plan.
(c)Represents income resulting from the CARES Act offset by additional cleaning and disinfecting expenses and contract termination fees for canceled events.
(d)Primarily representsintegration costs related to the CEO transition.
(e)Represents costscompleted acquisitions, amortization of inventory step-up associated with acquired entities, loss on disposal of fixed assets related to acquired businesses, and changes in fair value of contingent consideration.
(c)Costs incurred were the result of adopting restructuring plans to reduce headcount, contract termination, reorganize management structure and consolidate certain facilities.
(d)Includes compensation expense resulting from awards granted under our equity-based compensation plans. The year ended December 31, 2023 includes the reversal of equity compensation expenses totaling $3.8 million related to the transition of our executive leadership.
(e)Financial restructuring costs include advisory fees and debt amendment related costs.
(f)Represents a shiftnon-cash impairment charge for intangible assets attributable to our Wound Business due to its divestiture during the year ended December 31, 2023. Represents asset impairment charges on Trice Medical, Inc. for the year ended December 31, 2022.
(g)Represents a non-cash impairment charge due to the decline in the Company’s market capitalization.
(h)Represents the loss on the disposal of the Wound Business.
(i)Other items primarily includes charges associated with strategic transactions, such as potential acquisitions or divestitures, projects associated with improving business capabilities/efficiencies and costs attributable to MOTYS. During the 2022 fiscal year, prior to obtaining the results from directour Phase 2 trial, we elected to discontinue the development of MOTYS to focus our resources on other priorities, including the integration of our acquisitions and our expanded R&D and product development portfolio we inherited with these acquisitions. We incurred $1.0 million and $4.3 million in costs during the years ended December 31, 2023 and 2022, respectively, related to MOTYS. No further costs are expected on MOTYS.
Net sales
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| U.S. | | | | | | | |
| Pain Treatments | $ | 197,954 | | | $ | 194,830 | | | $ | 3,124 | | | 1.6 | % |
| Restorative Therapies | 116,851 | | | 134,214 | | | (17,363) | | | (12.9 | %) |
| Surgical Solutions | 135,055 | | | 126,207 | | | 8,848 | | | 7.0 | % |
| Total U.S. net sales | 449,860 | | | 455,251 | | | (5,391) | | | (1.2 | %) |
| | | | | | | |
| International | | | | | | | |
| Pain Treatments | 22,847 | | | 21,495 | | | 1,352 | | | 6.3 | % |
| Restorative Therapies | 20,222 | | | 20,420 | | | (198) | | | (1.0 | %) |
| Surgical Solutions | 19,416 | | | 14,951 | | | 4,465 | | | 29.9 | % |
| Total International net sales | 62,485 | | | 56,866 | | | 5,619 | | | 9.9 | % |
| | | | | | | |
| Total net sales | $ | 512,345 | | | $ | 512,117 | | | $ | 228 | | | — | % |
U.S.
Net sales decreased $5.4 million, or 1.2%, compared to the prior year. Changes by major product groups were: (i) Pain Treatments—$3.1 million increase due to volume partially offset with lower reported ASP during most of 2023, thereby generating lower reimbursement levels; (ii) Restorative Therapies—$17.4 million net sales decrease due primarily to the divestiture of our Wound Business; and (iii) Surgical Solutions—$8.8 million net sales increase due to volume growth.
International
Net sales increased $5.6 million, or 9.9%, due to volume growth in Pain Treatments and Surgical Solutions, partially offset with a volume decline in Restorative Therapies from advanced rehabilitation.
Gross profit and gross margin
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| U.S. | $ | 294,366 | | | $ | 296,782 | | | $ | (2,416) | | | (0.8 | %) |
| International | 33,827 | | | 34,298 | | | (471) | | | (1.4 | %) |
| Total | $ | 328,193 | | | $ | 331,080 | | | $ | (2,887) | | | (0.9 | %) |
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | |
| 2023 | | 2022 | | Change | | | | | | |
| U.S. | 65.4 | % | | 65.2 | % | | 0.2 | % | | | | | | |
| International | 54.1 | % | | 60.3 | % | | (6.2 | %) | | | | | | |
| Total | 64.1 | % | | 64.6 | % | | (0.5 | %) | | | | | | |
U.S.
Gross profit decreased $2.4 million, or 0.8%, primarily due to the divestiture of our Wound Business, the decrease in selling price within Pain Treatments and an indirect distribution modelincrease in our cost of sales due to inventory write-offs. The change in gross margin included a decrease from product mix and selling price, offset with a decrease in rebates and the absence of amortization of acquisition related assets in 2022 compared with 2023.
International business
Gross profit remained consistent with prior period. Gross margin decreased due to product mix.
Selling, general and administrative expense
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Selling, general and administrative expense | $ | 303,879 | | | $ | 332,134 | | | $ | (28,255) | | | (8.5 | %) |
Selling, general and administrative expense decreased $28.3 million, or 8.5%, primarily due to a decrease in equity-based compensation of $14.5 million due to employee turnover and the decline in stock price and cost saving initiatives. These initiatives resulted in a decrease of $7.3 million in other administrative and marketing related costs and a decline of $6.3 million in bad debt due to increased collections.
Research and development expense
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Research and development expense | $ | 13,446 | | | $ | 23,854 | | | $ | (10,408) | | | (43.6 | %) |
Research and development expense decreased $10.4 million, or 43.6%, primarily due to: (i) a decrease of $8.2 million in consulting costs; (ii) a decline of $1.0 million in supplies expense due to cost reduction efforts; and (iii) a decrease of $0.4 million in equity-based compensation due to employee turnover.
Restructuring costs
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Restructuring costs | $ | 840 | | | $ | 6,779 | | | $ | (5,939) | | | (87.6 | %) |
Restructuring costs for the year ended December 31, 2023 primarily included costs incurred as a result of an initiative to align the Company’s organizational and management cost structure to improve performance. Various international subsidiariesprofitability and cash flow through headcount reduction and reducing third-party related costs. Costs incurred for the year ended December 31, 2022 included costs to reduce headcount, reorganize management structure and to consolidate certain facilities for acquired businesses.
Change in fair value of contingent consideration
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Change in fair value of contingent consideration | $ | 719 | | | $ | 1,102 | | | $ | (383) | | | (34.8 | %) |
The fair value of contingent consideration during year ended December 31, 2023 remained consistent with the prior year comparable period. The activity for both periods relates to contingent consideration associated with the acquisition of Bioness in March 2021.
Depreciation and amortization
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Depreciation and amortization | $ | 8,842 | | | $ | 9,748 | | | $ | (906) | | | (9.3 | %) |
Depreciation and amortization decreased during year ended December 31, 2023 compared with the prior year primarily due to: (i) the impairment of intellectual property intangible assets associated with the Wound Business, which was divested during the second quarter of 2023; and (ii) a decline in amortization for certain customer relationships that became fully amortized during the first quarter of 2023. These decreases were dissolvedpartially offset with an increase in depreciation expense for information technology assets, right-of-use assets associated with finance leases and or merged into other BV LLC entities.consignment and demo assets.
(f)Foreign currency impact represents realizedImpairment of assets
Our decision to divest the Wound Business required us to evaluate whether certain of its assets were impaired. We recorded a $78.6 million non-cash impairment charge as a result of this evaluation to reduce the intangible assets to their fair values less costs to sell. The fair value of intangibles of the Wound Business was determined based on the consideration offered for the Wound Business.
Impairment of goodwill
We concluded that the carrying value of the U.S. reporting unit exceeded its fair value and unrealized gainsrecorded a non-cash goodwill impairment charge of $189.2 million during the year ended December 31, 2022, of which $124.7 million was recorded in the impairment of assets and losses from fluctuations$64.5 million in foreign currency and is includedloss on discontinued operations, net of tax, respectively, within other (income) loss in the consolidated statements of operations and comprehensive income (loss).
(g)Represents our share in the losses of CartiHeal for the year ended December 31, 2020.
(h)Other non-recurring items in 2020 includes settlement and legal costs of $1.9 million with the OIG. The remaining activities in 2020 and the balance in 2019 are primarily comprised of charges associated with potential strategic transactions, such as potential acquisitions and preparing to become a public company, primarily accounting and legal fees.
Net sales
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| U.S. | $ | 293,697 | | | $ | 305,072 | | | $ | (11,375) | | | (3.7 | %) |
| International | 27,464 | | | 35,069 | | | (7,605) | | | (21.7 | %) |
| Net Sales | $ | 321,161 | | | $ | 340,141 | | | $ | (18,980) | | | (5.6 | %) |
U.S.
Net sales decreased $11.4 million, or 3.7%, for year ended December 31, 2020, compared to the year ended December 31, 2019. The changes in net sales by vertical are as follows:
| | | | | |
•Minimally invasive fracture treatment
| ($11.5) million |
•OA joint pain treatment and joint restoration
| ($6.3) million |
•Bone graft substitutes
| $6.4 million |
income.
Minimally invasive fracture treatment decreased primarily due to sales volume declines resulting from the disruption caused by the COVID-19 pandemic. OA joint pain and joint preservation decreased primarily due to the COVID-19 pandemic as well as more treatments being sold under contracts with major insurers at lower prices, partially offset by sales volume growth. These decreases were also offset by sales volume growth within our BGS vertical.Loss on disposals
International
Net sales decreased $7.6 million, or 21.7%, for the year ended December 31, 2020, compared to the year ended December 31, 2019, primarily due to a decline in order volumes due to the disruption caused by the COVID-19 pandemic.
Gross profit and gross margin
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| Gross profit | | | | | | | |
| U.S. | $ | 214,572 | | | $ | 224,957 | | | $ | (10,385) | | | (4.6 | %) |
| International | 18,947 | | | 24,249 | | | (5,302) | | | (21.9 | %) |
| Total | $ | 233,519 | | | $ | 249,206 | | | $ | (15,687) | | | (6.3 | %) |
| | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | Change |
| | 2020 | | 2019 | |
| Gross margin | | | | | | |
| U.S. | | 73.1 | % | | 73.7 | % | | (0.6 | %) |
| International | | 69.0 | % | | 69.1 | % | | (0.1 | %) |
| Consolidated | | 72.7 | % | | 73.3 | % | | (0.6 | %) |
U.S.
Gross profit decreased $10.4 million, or 4.6%, for the year ended December 31, 2020, compared to the year ended December 31, 2019, primarily due to the decline in net sales above described. Manufacturing variances created by maintaining consistent production levels in a period of low demand coupled with idle time attributed to the COVID-19 pandemic caused a slight decrease of 0.6% in gross margin during 2020.
International
Gross profit decreased $5.3 million, or 21.9%, for the year ended December 31, 2020, compared to the year ended December 31, 2019, primarily due to the decrease in sales from the disruption caused by COVID-19 pandemic.
Selling, general and administrative expense
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| Selling, general and administrative expense | $ | 193,078 | | | $ | 198,475 | | | $ | (5,397) | | | (2.7 | %) |
Selling, general and administrative expense declined $5.4 million, or 2.7%, for the year ended December 31, 2020, compared to the year ended December 31, 2019, primarily due to:
| | | | | |
•COVID-19 related decreases, including declines in travel and meetings from doing business virtually, lower compensation related expenses as well as various cost-reduction initiatives
| $9.3 million |
•Lower legal and accounting expenses, which were higher in 2019 due to the OIG matter discussed in Note 12. Commitment and contingencies within Part II, Item 8. Financial Statements and Supplementary data
| $5.8 million |
These items were partially offset by the following increases:
| | | | | |
•Succession and transition charges associated with the transition to our new Chief Executive Officer
| $5.6 million |
•Costs and services related to strategic transactions, product recall expenses and other non-recurring charges
| $4.1 million |
Research and development expense
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| Research and development expense | $ | 11,202 | | | $ | 11,055 | | | $ | 147 | | | 1.3 | % |
Research and development expense increased $0.1 million, or 1.3%, for the year ended December 31, 2020, compared to the year ended December 31, 2019, primarily due to costs relating to the development agreement for MOTYS being almost entirely offset by cost reduction initiatives undertaken as a result of the COVID-19 pandemic.
Depreciation and amortization
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| Depreciation and amortization | $ | 7,439 | | | $ | 7,908 | | | $ | (469) | | | (5.9) | % |
Depreciation and amortizationThe loss on disposals during the year ended December 31, 2020 remained consistent2023 resulted from $1.5 million in working capital adjustments associated with the year ended December 31, 2019, as it slightly decreased $0.5sale of our Wound Business and a $2.0 million or 5.9%.loss on fixed assets disposed of during the integration of acquisitions.
Other (income) expense (income)
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| Interest expense | $ | 9,751 | | | $ | 21,579 | | | $ | (11,828) | | | (54.8 | %) |
| Other (income) loss | $ | (4,428) | | | $ | (75) | | | $ | (4,353) | | | NM |
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Interest expense, net | $ | 40,676 | | | $ | 12,021 | | | $ | 28,655 | | | 238.4 | % |
| Other (income) expense | $ | (1,290) | | | $ | 9,770 | | | $ | (11,060) | | | (113.2 | %) |
Interest expense, decreased $11.8net increased $28.7 million or 54.8%, for the year ended December 31, 2020, compared to the year ended December 31, 2019, primarilydue to: (i) an increase in interest of $16.0 million due to decreased debthigher interest resulting from refinancing our debt in December 2019 as well asrates; (ii) the decline in interest rates. This decrease was partially offset with an increaseabsence of $1.6$6.4 million primarilybenefit resulting from the declinechange in fair value of our interest rate swap.swap, which was discontinued during the fourth quarter of 2022; (iii) an increase of $3.9 million due to higher margin rates on our Term Loan; (iv) a $0.9 million increase in amortization of discount and deferred financing costs; and (v) an increase of $0.8 million in lease finance interest expense.
Other income during year ended December 31, 2020 was primarily the result of receiving Provider Relief Fund payments of approximately $4.1(income) expense changed $11.1 million in the aggregate pursuantdue to the CARES Act.
Income tax expense
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| Income tax expense | $ | 1,192 | | | $ | 1,576 | | | $ | (384) | | | (24.4 | %) |
| Effective tax rate | 7.5 | % | | 16.3 | % | | | | (8.8 | %) |
Income tax expense decreased $0.4(benefit), net
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Income tax expense (benefit), net | $ | 85 | | | $ | (44,374) | | | $ | 44,459 | | | (100.2) | % |
| Effective tax rate | 0.1 | % | | 23.5 | % | | | | (23.4 | %) |
The $44.5 million or 24.4%, for the year ended December 31, 2020, compared to the year ended December 31, 2019, primarily due to the change in income from continuing operationstaxes was driven by movement in net losses before income taxes. Our change in effective tax rate istaxes and the resultimplementation of a full valuation allowance during the first quarter of 2023.
Noncontrolling interest
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Continuing LLC Owner | $ | 39,395 | | | $ | 54,240 | | | $ | (14,845) | | | (27.4 | %) |
| Other noncontrolling interest | — | | | 447 | | | (447) | | | (100.0 | %) |
| Total | $ | 39,395 | | | $ | 54,687 | | | $ | (15,292) | | | |
Subsequent to the IPO and related transactions, we are the sole managing member of BV LLC’s pass-through structure for U.S. income tax purposes, while being treated as taxableLLC in certain stateswhich we own 80.0%. We have a majority economic interest, the sole voting interest in, and various foreign jurisdictions as well as for certain subsidiaries.control the management of BV LLC. As a result, we consolidate the financial results of BV LLC and report a non-controlling interest representing the 20.0% that is owned by the Continuing LLC Owner.
Segment Adjusted EBITDA
Adjusted EBITDA for each of our reportable segments is as follows:
| | Years Ended December 31, | | Change |
| Years Ended December 31, | | | Years Ended December 31, | | Change |
| (in thousands, except for percentage) | (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % | (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| U.S. | U.S. | $ | 69,252 | | | $ | 71,673 | | | $ | (2,421) | | | (3.4 | %) | U.S. | $ | 78,668 | | | $ | | $ | 56,513 | | | $ | | $ | 22,155 | | | 39.2 | | 39.2 | % |
| International | International | $ | 3,191 | | | $ | 7,515 | | | $ | (4,324) | | | (57.5 | %) | International | $ | 10,194 | | | $ | | $ | 12,097 | | | $ | | $ | (1,903) | | | (15.7) | | (15.7) | % |
U.S.
Adjusted EBITDA decreased $2.4increased $22.2 million, or 3.4%39.2%, during the comparable time periods. The decline was the result of decreased gross profit, excluding depreciation and amortization in cost of sales,primarily due to the decreasecost saving initiatives, a reduction in sales primarily resulting from the economic impact of COVID-19, which also led to lower expenses resulting from doing business virtually, lower compensation relatedbad debt and declines in administrative and travel expenses as well as various other cost-reduction initiatives.
previously discussed, partially offset with lower gross profit.International
Adjusted EBITDA decreased $4.3$1.9 million or 57.5%, during the comparable time periods. The decline was the result of decreased gross profit, excluding depreciation and amortization in cost of sales, resulting from the decrease in sales primarily due to the economic impact of COVID-19, which was partially offset by lower expenses resulting from doing business virtually, reducedincreased compensation related expensescosts and various other cost-reduction initiatives.a slight decline in gross profit.
Liquidity and Capital Resources
OverviewSources of liquidity
Our principal liquidity needs have historically been for acquisitions, working capital, research and development, clinical trials, and capital expenditures. We expect these needs to continue as we develop and commercialize new products and further our expansion into international markets.
We believe thathave implemented previously announced restructuring initiatives to enhance our existing cashcurrent financial position and cash equivalents, borrowing capacity under our revolving credit facility, cash flow from operations,sources of liquidity. These restructuring efforts delivered $9.0 million to $10.0 million in cost savings on an annualized basis. Refer to Item 8. Financial Statements and net proceeds from our IPO will be enoughSupplementary Data—Notes to meet our anticipated cash requirementsthe Consolidated Financial Statements—Note 10. Restructuring costs for at least the next twelve months. We may require additional liquidity as we continue to execute our business strategy. Negative impacts to our liquidity would include a decline in sales of our products, including declines due to changes in our customers’ ability to obtain third-party coverage and reimbursement for procedures that utilize our products, increased pricing pressures resulting from intensifying competition andfurther details regarding these cost increases, as well as general economic and industry factors. cutting efforts.
We anticipate that to the extent that we require additional liquidity, we will obtain funding through additional equity financings or the incurrence of other indebtedness additional equity financings or a combination of these potential sources of liquidity. We may explore divestiture opportunities for non-core assets to improve our liquidity position. In addition, we may raise additional funds to finance future cash needs through receivables or royalty financings or corporate collaboration and licensing arrangements. If we raise additional funds by issuing equity securities or convertible debt, our stockholders will experience dilution. The covenants under the Amended 2019 Credit Agreement limit our ability to obtain additional debt financing. Debt financing, if allowed under the Amended 2019 Credit Agreement and if available, would result in increased fixed payment obligations and maymight involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, or making capital expenditures. If we raise additional funds through collaboration and licensing arrangements with third parties, it maymight be necessary to relinquish valuable rights to our products, future revenue streams or product candidates, or to grant licenses on terms that maymight not be favorable to us. The covenants under our credit agreement limit our ability to obtain additional debt financing. We cannot be certain that additional funding will be available on acceptable terms, or at all. Any failure to raise capital in the future couldmight have a negative impact on our financial condition and our ability to pursue our business strategies.
Initial public offering
On February 16, 2021, in connection with our IPO, we issued and sold 9,200,000 shares of our Class A common stock at a price to the public of $13.00 per share, resulting in gross proceeds to us of approximately $119.6 million, before deducting the underwriting discount, commissions and estimated offering expenses payable by us.
We are Bioventus Inc. is a holding company and havehas no material assets other than itsthe ownership of LLC Interests in BV LLC and has no independent means of generating revenue. The limited liability company agreement of BV LLC provides for the payment of certain distributions to the Continuing LLC Owner and us in amounts sufficient to cover the income taxes imposed on such members with respect to the allocation of taxable income from BV LLC as well as to cover our obligations under the Tax Receivable Agreement (TRA). Additionally, in the event we declare any cash dividend, we intend to cause BV LLC to make distributions to us, in an amount sufficient to cover such cash dividends declared by us. Deterioration in the financial condition, earnings, or cash flow of BV LLC and its subsidiaries for any reason could limit or impair their ability to pay such distributions. In addition, the terms of our financing arrangements, including the Amended 2019 Credit Agreement, contain covenants that may restrict BV LLC and its subsidiaries from paying such distributions, subject to certain exceptions. Further, BV LLC is generally prohibited under Delaware law from making a distribution to a member to the extent that, at the time of the distribution, after giving effect to the distribution, liabilities of BV LLC (with certain exceptions), as applicable, exceed the fair value of its assets. Subsidiaries of BV LLC are generally subject to similar legal limitations on their ability to make distributions to BV LLC. If we do not haveBioventus Inc., as the managing member, causes BV LLC to make cash distributions to the owners of LLC Interests in an amount sufficient funds to pay taxes or other liabilities or to(i) fund our operations, we may have to borrow funds, which could materially adversely affect our liquiditytax obligations in respect of allocations of taxable income from BV LLC; and financial condition and subject us to various restrictions imposed by any such lenders. To the extent that we are unable to make(ii) cover Bioventus Inc. operating expenses, including payments under the TRATRA.
Future cash requirements
The following table summarizes certain estimated future cash requirements under our various contractual obligations committed to as of December 31, 2023 in total and disaggregated into current and long-term obligations.
| | | | | | | | | | | | | | | | | |
| (in thousands) | Current | | Long-Term | | Total |
Long-term debt(a) | $ | 27,848 | | | $ | 354,600 | | | $ | 382,448 | |
Interest payments on long-term debt obligations(a) | 37,615 | | | 58,585 | | | 96,200 | |
Lease liabilities(b) | 4,816 | | | 20,959 | | | 25,775 | |
Purchase commitments(c) | 35,146 | | | 12,973 | | | 48,119 | |
| $ | 105,425 | | | $ | 447,117 | | | $ | 552,542 | |
(a)Refer to Item 8. Financial Statements and Supplementary Data—Notes to the Consolidated Financial Statements—Note 5. Financial instruments in this Annual Report for any reason, such payments generally will be deferredfurther information regarding long-term debt obligations.
(b)Refer to Item 8. Financial Statements and will accrue interest until paid; provided, however, that nonpaymentSupplementary Data—Notes to the Consolidated Financial Statements—Note 12. Commitments and contingencies in this Annual Report for a specified period may constitute a material breach of a material obligation under the TRAfurther information regarding operating and therefore accelerate payments due under the TRA.
finance lease liabilities.
In addition,(c)Amounts that are contractually committed to as of December 31, 2023 related to multi-year exclusive supply agreements. Generally, our purchase obligations under these supply agreements are based on forecasted requirements, subject in some cases to an annual contractual minimum.
Other cash requirements
We enter into contracts in the normal course of business with various third parties for development, collaboration and other services for operating purposes. These contracts provide for termination upon notice. Payments due upon cancellation generally consist only of payments for services provided or expenses incurred, including non-cancellable obligations of our service providers, up to the date of cancellation. Certain agreements include contingent events that upon occurrence would require payment. For information regarding Commitments and Contingencies, refer to Item 8. Financial Statements and Supplementary Data in this Annual Report.
Tax Receivable Agreement
The BV LLC Agreement provides for the payment of certain distributions to the Continuing LLC Owner in amounts sufficient to cover the income taxes imposed with respect to the allocation of taxable income from BV LLC as well as obligations within the TRA. Under the TRA, we are required to make cash payments to the Continuing LLC Owner equal to 85% of the tax benefits, if any, that we actually realize (or in certain circumstances are deemed to realize), as a result of (1) increases in the tax basis of assets of BV LLC resulting from (a) any future redemptions or exchanges of LLC Interests, described under Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Bioventus LLC Agreement-LLC Interest Redemption Right, and (b) certain distributions (or deemed distributions) by BV LLC and (2) certain other tax benefits arising from payments under the TRA. We expect the amount of the cash payments required to be made under the TRA will be significant. The actual amount and timing of any payments under the TRA will vary depending upon a number of factors, including the timing of redemptions or exchanges by the Continuing LLC Owner, the amount of gain recognized by the Continuing LLC Owner, the amount and timing of the taxable income we generate in the future, and the federal tax rates then applicable. Any payments made by us to the Continuing LLC Owner under the TRA will generally reduce the amount of overall cash flow that might have otherwise been available to us. To the extent that we are unable to make payments under the TRA for any reason, such payments generally will be deferred and will accrue interest until paid; provided, however, that nonpayment for a specified period may constitute a material breach of a material obligation under the TRA and therefore accelerate payments due under the TRA.
CashIndebtedness
Our 2019 Credit Agreement (as amended on August 29, 2021 and cash equivalents,October 29, 2021), and in effect from October 29, 2021 to July 11, 2022, consisted of a $360.8 million term loan (“Term Loan”) and a $50.0 million revolving credit facility (the “Revolver”). On July 11, 2022, we amended the 2019 Credit Agreement in conjunction with the CartiHeal Acquisition to provide for an incremental $80.0 million term loan facility (together with the Term Loan, the “Term Loan Facilities”) to be used in part to fund the CartiHeal Acquisition.
The Company was not in compliance with certain of its covenants under the 2019 Credit Agreement in effect as of December 31, 2020, totaled $86.8 million compared2022. As a result, on March 31, 2023, we entered into another amendment to $64.5 millionthe 2019 Credit Agreement to, among other things, modify certain financial covenants, waive the noncompliance at December 31, 2019. Changes2022 and modify interest rates applicable to borrowings under the agreement. We were in cash arecompliance with the financial covenants as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2020 | | 2019 | | $ | | % |
| Consolidated statement of cash flow data: | | | | | | | |
| Net cash provided by (used in): | | | | | | | |
| Operating activities from continuing operations | $ | 72,199 | | | $ | 42,545 | | | $ | 29,654 | | | 69.7 | % |
| Investing activities from continuing operations | (20,672) | | | (7,912) | | | (12,760) | | | 161.3 | % |
| Financing activities | (29,569) | | | (10,951) | | | (18,618) | | | 170.0 | % |
| Discontinued operations | (228) | | | (1,832) | | | 1,604 | | | (87.6) | % |
Effect of exchange rate changes on cash and
cash equivalents | 589 | | | (104) | | | 693 | | | NM |
| Net change in cash and cash equivalents | $ | 22,319 | | | $ | 21,746 | | | $ | 573 | | | |
Operating Activities
Cash flows from operating activities from continuing operations increased $29.7 million during the year endedof December 31, 2020 compared to year ended December 31, 2019 due to collections on accounts receivables staying strong while selling, general and administrative expenses declined, partially offset by the timing of rebate payments. We experienced lower travel expense payments resulting from the near halting of all travel due to the COVID-19 pandemic, a reduction in compensation related expenses due to the decline in sales and increased cash savings from cost cutting measures. We also received stimulus payments from government entities while we purchased less inventory due to the decline in sales. In addition, our interest expense was significantly lower during the year ended December 31, 2020 due to the December 2019 refinancing.
Investing Activities
Cash flows used in investing activities increased $12.8 million during the year ended December 31, 2020 compared to year ended December 31, 2019 primarily due to $16.6 million in payments for the 2020 CartiHeal investments and an increase of $1.8 million in capital expenditures due to software and technology upgrades. These investing cash outflows were partially offset by the absence of payments for distribution rights in 2020 compared to $6.0 million for the purchases of distribution rights during 2019.
Financing Activities
Cash flows used in financing activities increased $18.6 million during the year ended December 31, 2020 compared to the year ended December 31, 2019 primarily due to a $10.7 million increase in distribution to members2023 as well as the $6.1 million increase in debt payments.
Other
For information regarding Commitments and Contingencies, refer to Note 12. Commitment and contingencies in Part II, Item 8. Financial Statements and Supplementary Data in this Annual Report for further information regarding other matters.
Indebtedness
On December 6, 2019, we entered intostated within the 2019 Credit Agreement. TheAgreement then in effect.
On January 18, 2024 (the “Closing Date”), we further amended the 2019 Credit Agreement (collectively, with the August 2021, October 2021, July 2022 and March 2023 amendments, the “Amended 2019 Credit Agreement”), to modify certain financial covenants under the 2019 Credit Agreement.
With respect to Term Loan Facilities and the Revolver outstanding as of the Closing Date, we may elect either SOFR or Base Rate interest rate options for the entire amount or certain portions of the loans and have interest rates equal to a formula driven base interest rate plus a margin, tied to a leverage ratio. The leverage ratio is comprisedthe ratio of our $200.0 million term loandebt to Consolidated Adjusted EBITDA as defined in the Amended 2019 Credit Agreement for four consecutive quarters as of the end of each period. Pursuant to the Amended 2019 Credit Agreement, the margin at each applicable leverage ratio will be increased by 1.00% per annum. SOFR loans and our $50.0 million revolving credit facility.base rate loans had a margin of 3.25% and 2.25%, respectively, subsequent to July 11, 2022 and prior to March 31, 2023. As of the March 2023 amendment, SOFR loans and base rate loans had a margin of 4.25% and 3.25%, respectively. All obligations under the Amended 2019 Credit Agreement are guaranteed by the Company and certain of our wholly owned domestic subsidiaries and secured bywhere substantially all our and the guarantors’ assets. assets of the Company collateralize the obligations.
The term loan and revolving credit facilityTerm Loan Facilities will mature on October 29, 2026. The Revolver will mature on October 29, 2025. The capacity on the Revolver was reduced to $43.2 million at December 6, 2024. 31, 2023 and will be further reduced by $5.0 million at June 30, 2024 in accordance with the Amended 2019 Credit Agreement.
The Amended 2019 Credit Agreement contains various restrictivecustomary affirmative and negative covenants, including a quarterly covenant notthose related to exceed a consolidated leverage ratio of 3.50 to 1.00financial reporting and an interest coverage ratio of 3.00:1.00 for the prior four consecutive quarters. The leverage and interest coverage ratios are basednotification, restrictions on Consolidated EBITDA as defined in the 2019 Credit Agreement, which includes several differences from Adjusted EBITDA as calculated in this Annual Report. Consolidated EBITDA as defined in the 2019 Credit Agreement permits, among other things, the exclusion of (1) certain extraordinary, unusual and/or non-recurring expenses, some of which are subject to an aggregate cap, including but not limited to severance, acquisitions, dispositions, debt refinancing/amendment and IPO-related, (2) foreign currency gains/losses recognized in the statement of operations and (3) franchise, excise and property taxes recognized in the statement of operations. The restrictive covenants include limitations on (1) the declaration or payment of certain distributions on or in respect of ourBioventus LLC’s equity interests, (2) restrictions on acquisitions, investments and certain other payments, (3) limitations on the incurrence of new indebtedness, (4) limitations on transfers, sales and other dispositions of assets of Bioventus LLC and (5)its subsidiaries, as well as limitations on making changes to ourthe business and organizational documents. Asdocuments of Bioventus LLC and its subsidiaries. Financial covenant requirements include: (i) a maximum debt leverage ratio of not greater than 5.65 to 1.00 for the testing period ending March 31, 2024, 5.00 to 1.00 for the testing period ending June 30, 2024, 4.75 to 1.00 for the testing period ending September 30, 2024, 4.50 to 1.00 for the testing period ending December 31, 2020, we complied2024, 4.25 to 1.00 for testing period ending March 31, 2025, 4.00 to 1.00 for testing period ending June 30, 2025 and each testing period thereafter, and, beginning with allthe testing period ending March 31, 2025, to be subject to a temporary increase to 4.50 to 1.00 upon certain events, and (ii) an interest coverage ratio not less than 1.75 to 1.00 for the testing period ending March 31, 2024, 1.75 to 1.00 for the testing period ending June 30, 2024, 1.75 to 1.00 for the testing period ending September 30, 2024, 2.00 to 1.00 for the testing period ending December 31, 2024, 2.00 to 1.00 for the testing period ending March 31, 2025, 2.25 to 1.00 for the testing period ending June 30, 2025, 2.50 to 1.00 for the testing period ending September 20, 2025 and 3.00 to 1.00 for the testing period ending December 31, 2025 and each testing period thereafter. In addition, during the period commencing on the Closing Date and ending upon the satisfaction of certain conditions occurring not prior to October 29, 2025, the Company will be subject to certain additional requirements and covenants, underincluding a requirement to maintain Liquidity (as defined in the Amended 2019 Credit Agreement and there was $190.0Agreement) of not less than $10.0 million of outstanding borrowings under the term loan. We have one nominal outstanding letter of credit. In March 2020, as a precautionary measure and in order to increase our cash position and preserve financial flexibility in view of the uncertainty resulting from the COVID-19 pandemic, we drew down $49.0 million on our revolving credit facility. On September 24, 2020, we repaid all borrowings outstanding under our revolving credit facility. Our revolving credit facility had $50.0 million in borrowing capacity asend of December 31, 2020.each calendar month during such period.
Off-balance sheet arrangements
We do not have any off-balance sheet arrangements.
Contractual Obligations
Our contractual obligations as of December 31, 2020 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Payments Due by Period |
| Contractual Obligations (in thousands) | | Less than 1 year | | 1-3 Years | | 3-5 Years | | More than 5 years | | Total |
| Long-term debt obligations | | $ | 15,000 | | | $ | 35,000 | | | $ | 140,000 | | | $ | — | | | $ | 190,000 | |
| Interest on long-term debt obligations | | 4,606 | | | 8,073 | | | 3,139 | | | — | | | 15,818 | |
| Operating lease obligations | | 1,960 | | | 3,955 | | | 4,232 | | | 5,921 | | | 16,068 | |
| Purchase obligations | | 19,655 | | | 25,920 | | | 25,920 | | | — | | | 71,495 | |
| | $ | 41,221 | | | $ | 72,948 | | | $ | 173,291 | | | $ | 5,921 | | | $ | 293,381 | |
The table above does not include certain obligations as follows:
•commitments under our multi-year exclusive supply agreements for our OA products except for those amounts that are contractually committed as of December 31, 2020. Our purchase obligations under these supply agreements are generally based upon our forecasted requirements, subject in some cases to a contractual minimum per annum;
•commitments under the Development Agreement with MTF, the Option and Equity Purchase Agreement with CartiHeal and its shareholders and the Collaboration Agreement with Harbor, for which the relevant contingent events requiring a payment under the respective agreements have not yet occurred; and
•future milestone payments pursuant to the Development Agreement with MTF, the Option and Equity Purchase Agreement with CartiHeal and its shareholders, the Collaboration Agreement with Harbor and the amended and restated license agreement, or the Q-Med License Agreement, with Q-Med and NSH, as the payment obligations under these agreements are contingent upon future events and we are unable to estimate the timing or likelihood of achieving these milestones or generating future product sales.
Recent accounting pronouncements
Refer to Note 2. Summary of significant accounting policies in Part II, Item 8. Financial Statements and Supplementary DataData—Notes to the Consolidated Financial Statements—Note 5. Financial instruments in this Annual Report for further informationdetails on the Company’s indebtedness.
Information regarding recently adoptedcash flows
Cash, cash equivalents and proposed accounting pronouncements.restricted cash as of December 31, 2023 totaled $37.0 million, compared to $30.2 million as of December 31, 2022. The change in cash was primarily due to the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| (in thousands, except for percentage) | 2023 | | 2022 | | $ | | % |
| Cash flows from continuing operations: | | | | | | | |
| Net cash from operating activities | $ | 17,513 | | | $ | (11,407) | | | $ | 28,920 | | | (253.5 | %) |
| Net cash from investing activities | 27,313 | | | (11,595) | | | 38,908 | | | (335.6 | %) |
| Net cash from financing activities | (26,653) | | | 62,076 | | | (88,729) | | | (142.9 | %) |
| Net cash from discontinued operations | (13,675) | | | (106,971) | | | 93,296 | | | (87.2 | %) |
| Effect of exchange rate changes on cash | 629 | | | 521 | | | 108 | | | 20.7 | % |
| Net change in cash, cash equivalents and restricted cash | $ | 5,127 | | | $ | (67,376) | | | $ | 72,503 | | | (107.6 | %) |
Operating Activities
Net cash in operating activities from continuing operations increased $28.9 million, primarily due to lower employee compensation and cost reduction efforts. These inflows were partially offset with an increase in interest payments, taxes and inventory payments.
Investing Activities
Net cash flows in investing activities from continuing operations increased $38.9 million, primarily due to the $34.7 million receipt of cash resulting from the sale of the Wound Business, $2.7 million less in capital expenditures and $1.5 million less in other investments and acquisitions in 2023.
Financing Activities
Cash flows from financing activities decreased $88.7 million, primarily due to: (i) borrowings of $79.7 million in long-term debt to fund the acquisition of CartiHeal in 2022; (ii) an increase of $18.2 million in debt principal payments; and (iii) $5.0 million less in proceeds from the issuance of stock as there were significantly more option exercises in 2022. These financing outflows were partially offset with $15.0 million in net additional borrowings under our revolving credit facility in 2023.
Discontinued Operations
Net cash flows from discontinued operations in 2023 were primarily the result of $10.2 million in fees used to settle the CartiHeal disposition and $1.4 million in cash held by the CartiHeal entity at the time of disposal. Net cash flows from discontinued operations in 2022 primarily related to the $104.8 million purchase of CartiHeal.
Internal control over financial reportingRecently Issued Accounting Pronouncements
Internal control over financial reporting is a process designedRefer to provide reasonable assuranceItem 8. Financial Statements and Supplementary Data—Notes to the Consolidated Financial Statements—Note 2. Significant accounting policies for information regarding the reliability of financial reporting and the preparation ofaccounting pronouncements that may impact our financial statements in accordance with GAAP.
Presently, as a newly public company, our management is not required to perform an annual assessment of the effectiveness of our internal control over financial reporting. This requirement will first apply to our second Annual Report on Form 10-K. Our independent public registered accounting firm will first be required to attest to the effectiveness of our internal control over financial reporting for our Annual Report on Form 10-K for the first year we are no longer either an “emerging growth company” or a “non-accelerated filer,” as such terms are defined by Rule 12b-2 of the Exchange Act.
future periods.
Critical accounting policies and estimatesAccounting Estimates
The preparation of the consolidated financial statements requires us to make assumptions, estimates and judgments that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of sales and expenses during the reporting periods. Certain of our more critical accounting policies require the application of significant judgment by management in selecting the appropriate assumptions for calculating financial estimates. By their nature, these judgments are subject to an inherent degree of uncertainty. On an ongoing basis, we evaluate our judgments, including those related to inventories and the recoverability of long-lived assets and the fair value of our common stock.assets. We use historical experience and other assumptions as the basis for our judgments in making these estimates. Because future events and their effects cannot be determined with precision, actual results could differ significantly from these estimates. Any changes in these estimates will be reflected in our consolidated financial statements as they occur. Refer to Note 1. Organization and basis of presentation of financial information in Part II, Item 8. Financial Statements and Supplementary Data Data—Notes to the Consolidated Financial Statements—Note 2. Significant accounting policies for a further description of our significant accounting policies, however, we believe that the following accounting policies and estimates that are considered critical to our business in order to obtain a full understanding and to evaluate our reported financial results. The critical accounting policiesestimates addressed below reflect our most significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue recognition estimates
Sale of productsVariable consideration estimates
We derive revenue primarily from the sale of our OA joint pain treatment and joint preservation products, BGSs and minimally invasive fracture treatment. We sell these products directly to healthcare institutions, patients, distributors and dealers. We also enter into arrangements with pharmacy and health benefit managers that provide for privately negotiated rebates, chargebacks and discounts with respect to the purchase of our products. We recognize revenue generally at a point in time upon transfer of control of the promised product to customers in an amount that reflects the consideration we expect to receive in exchange for those products. We exclude from revenues taxes collected from customers and remitted to governmental authorities.authorities from revenues.
Revenues are recorded at the transaction price, which is determined as the contracted price net of estimates of variable consideration resulting from discounts, rebates, returns, chargebacks, contractual allowances, estimated third-party payer settlements, and certain distribution and administration fees offered in our customer contracts and other indirect customer contracts relating to the sale of our products. We establish reserves for the estimated variable consideration based on the amounts earned or eligible for claim on the related sales. Where appropriate, these estimates take into consideration a range of possible outcomes, which are probability-weighted for relevant factors such as our historical experience,experiences, current contractual requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. The amount of variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period. We regularly review all reserves and update them at the end of each reporting period as needed. There were no significant adjustments arising from the change in estimates of variable consideration for the years ended December 31, 20202023 and 2019.
OA joint pain treatment and joint preservation
Revenue from customers such as healthcare providers, distribution centers or specialty pharmacies is recognized at the point in time when control is transferred to the customer, typically upon shipment.
Distributor chargebacks
We have preexisting contracts with established rates with many of the distributors’ customers that require the distributors to sell our product at their established rate. We offer chargebacks to distributors who supply these customers with our products. We reduce revenue at the time of sale for the estimated future chargebacks. We record chargeback reserves as a reduction of accounts receivable and base the reserves on the expected value by using probability-weighted estimates of volume of purchases, inventory holdings and historical chargebacks requested for each distributor.
Discounts and rebates
We offer retrospective discounts and rebates linked to the volume of purchases which may increase at negotiated thresholds within a contract-buying period. We reduce revenue and record the reserve as a reduction to accounts receivable for the estimated discount and rebate at the most likely amount the customer will earn, based on historical buying trends and forecasted purchases.
Bone graft substitutes
The majority of our BGS product sales are through consignment inventory with hospitals, where ownership remains with us until the hospital performs a surgery and consumes the consigned inventory. We recognize the revenue when the surgery has been performed. The customer does not have control of the product until the customer consumes it, as we are able to require the return or transfer of the product to a third-party. An unconditional obligation to pay for the product does not exist until the customer consumes it.
Minimally invasive fracture treatment
We recognize revenue from third-party payers, such as governmental agencies, insurance companies or managed care providers, when we transfer control of the Exogen system to the patient, typically when the patient has accepted the product or upon delivery. We record this revenue at the contracted rate, net of contractual allowances at the time of sale, or an estimated price based on historical data and other available information for non-contracted payers. We record contractual allowances based on probability weighting historical data and collections. We recognize revenue from patients (self-pay and insured patients with coinsurance and deductible responsibilities) based on billed amounts giving effect to any discounts and implicit price concessions. Implicit price concessions represent differences between amounts billed and the amounts we expect to collect from patients, which considers historical collection experience and current market conditions.
Settlements with third-party payers for retroactive revenue adjustments due to audits, reviews or investigations are considered variable consideration and are included in the determination of the estimated transaction price using the most likely outcome method. These settlements are estimated based on the terms of the payment agreement with the payer, correspondence from the payer and historical settlement activity, including an assessment to ensure that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the retroactive adjustment is subsequently resolved. Estimated settlements are adjusted in future periods as adjustments become known (that is, new information becomes available), or as years are settled or are no longer subject to such audits, reviews and investigations. We are not aware of any claims, disputes or unsettled matters with any payer that would materially affect revenues for which we have not been adequately provided.
Product returns
We estimate the amount of returns and reduce revenue in the period the related product revenue is recognized. We record a liability for expected returns based on probability weighted historical data.2022.
Accounts receivable netallowances for credit losses
Accounts receivable, net are amounts billed and currently due from customers. We record the amounts due net of allowance for doubtful accounts. We maintain allowances for credit losses to provide for receivables we do not expect to collect. We base the allowance on an assessment of customer creditworthiness, historical payment experience, the age of outstanding receivables and other information as applicable. Collection of the consideration that we expect to receive typically occurs within 30 to 90 days of billing. We apply the practical expedient for contracts with payment terms of one year or less which does not consider the effects of the time value of money. Occasionally, we enter into payment agreements with patients that allow payment terms beyond one year. In those cases, the financing component is not deemed significant to the contract.
Contract assets
Contract assets consist of unbilled amounts resulting from estimated future royalties from an international distributor that exceeds the amount billed. Contract assets are included in prepaid and other current assets on the consolidated balance sheets.
Contract liabilities
Contract liabilities consist of customer advance payments or deposits and deferred revenue. Occasionally for certain international customers, we require payments in advance of shipping product and recognizing revenue resulting in contract liabilities. Contract liabilities are included in accrued liabilities on the consolidated balance sheets.
Shipping and handling
We classify amounts billed for shipping and handling as a component of net sales. The related shipping and handling fees and costs as well as other distribution costs are included in cost of sales. We have elected to recognize shipping and handling activities that occur after control of the related product transfers to the customer as fulfillment costs and are included in cost of sales.
Contract costs
We apply the practical expedient of recognizing the incremental costs of obtaining contracts as an expense when incurred as the amortization period of the assets that we otherwise would have recognized is one year or less. These incremental costs include our sales incentive programs for the internal sales force and third-party sales agents as the compensation is commensurate with annual sales activities. These costs are included in selling, general and administrative expense on the consolidated statements of operations and comprehensive income (loss).
Use of estimates
The preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities, at the date of the financial statements, as well as the reported amounts of revenues and expenses during the period. On an ongoing basis, management evaluates these estimates, including those related to contractual allowances and sales incentives, allowances for doubtful accounts, inventory reserves, goodwill and intangible assets impairment, useful lives of long lived assets, noncontrolling interest, fair value measurements, litigation and contingent liabilities, income taxes, and equity-based compensation. Management bases its estimates on historical experience, future expectations and other relevant assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from those estimates.
Fair value
We record certain assets and liabilities at fair value. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy that prioritizes the inputs used to measure fair value is described below. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. Assets and liabilities are categorized based on the lowest level that is significant to the valuation.
The three levels of inputs used to measure fair value are as follows:
•Level 1—Quoted prices in active markets for identical assets or liabilities;
•Level 2—Observable inputs other than quoted prices included within Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data; and
•Level 3—Unobservable inputs that are supported by little or no market data. This includes certain pricing models, discounted cash flow methodologies and similar techniques that use significant unobservable inputs.
Business combinations
We record identifiable assets acquired, liabilities assumed and any noncontrolling interest in an acquiree resulting from a business combination at their estimated fair values on the date of the acquisition. We generally have third-party valuations completed for intangible assets in a business combination using a discounted cash flow analysis, incorporating various assumptions. Goodwill represents the excess of the purchase price over the estimated fair value of the net assets acquired, including the amount assigned to identifiable intangible assets. The most significant estimates and assumptions inherent in a discounted cash flow analysis include the amount and timing of projected future cash flows, discount rate used to measure the risks inherent in the future cash flows, assessment of the asset’s life cycle, and competitive and other trends impacting the asset, including consideration of technical, legal, regulatory, economic and other factors. Each of these factors and assumptions can significantly affect the value of the intangible asset.
Acquired in-process research and development or (“IPR&D,&D”) is the fair value of projects for which the related products have not received regulatory approval and have no alternative future use and is capitalized as an indefinite-lived intangible asset. Due to inherent uncertainty related to research and development, actual results could differ materially from the assumptions used in the discounted cash flow model. Additionally, there are risks including, but not limited to, delay or failure to receive regulatory requirements to conduct clinical trials, required market clearances, or patent issuance, and that the research and development project does not result in a successful commercial product. Development costs incurred after the acquisition are expensed as incurred. Upon receipt of regulatory approval of the related technology or product, the indefinite-lived intangible asset is accounted for as a finite-lived intangible asset and amortized on a straight-line basis over its estimated useful life. If the research and development project is abandoned, the indefinite-lived asset is charged to expense.
Contingent consideration
We recognize contingent consideration liabilities resulting from business combinations at estimated fair value on the acquisition date. Contingent consideration liabilities are revalueddate, and at each subsequent toreporting period. Significant judgment is employed in determining the appropriateness of the estimates and assumptions as of the acquisition date with changesand in fair value recognized in earnings. Contingent paymentspost-acquisition periods. The Company initially values contingent consideration related to acquisitions consistbusiness combinations using a probability-weighted calculation of development, regulatory and commercial milestone payments, and are valued usingpotential payment scenarios discounted at rates reflective of the risks associated with the expected future cash flow techniques.flows. Significant estimates and assumptions required for these valuations include the probability of achieving regulatory approval under specified time frames, product sales projections under various scenarios and discount rates used to calculate the present value of the estimated payments. Changes inAfter the fair value ofinitial valuation, the Company will use its best estimate to measure contingent consideration liabilities result from changes in these estimatesat each subsequent reporting period. Gains and assumptions. Significant judgment is employed in determininglosses are recorded with selling, general and administrative expenses within the appropriatenessconsolidated statements of the estimatesoperations and assumptions ascomprehensive (loss) income.
Impairment of the acquisition dategoodwill and in post-acquisition periods.
Impairmentindefinite-lived intangible assets
We evaluate goodwill and other indefinite-lived intangible assets for impairment annually during the fourth quarter, or more frequently if events or changes in circumstances indicate that the asset might be impaired. Our reporting units are U.S. and International and we analyze each reporting unit separately in our goodwill impairment evaluation. We used independent third-party valuation specialists in 2020 and 2019 using year-to-date October data in each year to assist management in performing the annual review of goodwill for impairment. We also utilized valuation specialists in April 2020 as we believed COVID-19 represented a triggering event that might indicate impairment. The specialists assist management in the determination of fair value of reporting units based upon inputs and assumptions provided by management, which management uses for its impairment assessment. We analyze all other indefinite-lived intangible assets qualitatively to determine if it is more likely than not forthat an impairment to exist.exists. If we meet the criteria, we perform a quantitative analysis to determine if an impairment exists.
Goodwill Our reporting units are U.S. and International and we analyze each reporting unit separately in our impairment evaluations.
Our goodwill impairment process includes applying a quantitative impairment analysis whereto the fair value of the reporting unit and comparecomparing it to its carrying value (including goodwill).value. We used independent third-party valuation specialists in 2023 and 2022 using year-to-date October data in each year to assist management in performing our annual impairment evaluation. We determine the fair value of U.S. and International reporting units based primarily on an income approach, which incorporates the use of a discounted free cash flow analysis. The discounted free cash flow analysesanalysis is based on significant judgments, including the current operating budgets, estimated long-term growth projections and future forecasts for each reporting unit. We discount future cash flows based on a market comparable weighted average cost of capital rate for each reporting unit. The discount rates used in the discounted free cash flow analyses reflect the risks inherent in the expected future cash flows generated by the respective intangible assets. Market risk, industry risk and a small company premium has an impact on the discount rate. The value of each reporting unit is determined on a stand-alone basis from the perspective of a market participant and represents the price we estimate we would receive in a sale of the reporting unit in an orderly transaction between market participants at the measurement date. Significant judgments inherent in this analysis include estimating the amount and timing of future cash flows and the selection of appropriate discount rates, royalty rate and long-term growth rate assumptions. Changes in these estimates and assumptions could materially affect the determination of fair value for each reporting unit and for some of the reporting units and could result in an impairment charge, which could be material to our financial position and results of operations. There has been no
On November 8, 2022, due to a significant decline in the value of our Class A common stock, circumstances became evident that a possible impairment existed as of the third quarter balance sheet date. We concluded that the carrying value of the U.S. reporting unit exceeded its fair value. We recorded a non-cash goodwill impairment charge within the U.S. reporting unit for the year ended December 31, 2022. The impairment was recorded within impairment of goodwill on the consolidated statements of operations and comprehensive (loss) income. There were no goodwill impairment charges for the years ended December 31, 2023 and 2021. Refer to Item 8. Financial Statements and Supplementary Data—Notes to the Consolidated Financial Statements—Note 3. Balance sheet information.
Equity-based compensation
Equity-based compensation expense generated from the granting of restricted stock units represents the fair value of the stock measured at the market price on the date of grant. Restricted stock equity-based compensation expense is recognized over the vesting period.
The fair value of time-based stock options is determined using the Black-Scholes valuation model, with such value recognized as expense over the service period, net of actual forfeitures. Assumptions used in determining stock option fair value include risk-free interest rate, expected dividend yield, expected price volatility, expected life of stock options and weighted-average fair value of stock options granted. The expected term of the options granted is estimated using the simplified method. Expected volatility is based on the historical volatility of our goodwill related to ourpeers’ common stock. The risk-free interest rate is determined based upon a constant U.S. and International reporting units since our formation.
Equity compensationTreasury security rate with a contractual life that approximates the expected term of the option.
Prior to the IPO, the Companywe operated two equity-based compensation plans, the MIPManagement Incentive Plan (“MIP”) and the Phantom Plans, which allows our employees to share our future profit without granting any additional voting rights. Awards granted under the MIP and certain Profits Interest Plan (“Phantom Plan awards granted in 2015 and thereafter, or the 2015 Phantom Plan Units, are liability-classified. Those Phantom Plan awards granted from inception in 2012 and until the grant of the 2015 Phantom Plan Units, or the 2012 Phantom Plan Units, are equity-classified, as they do not contain a put option or other features requiring them to be liability-classified. Equity compensation includes compensation expense for all equity awards made to employees that are part of continuing operations and are based on estimated fair values as of the grant date for the 2012 Phantom Plan Units and period end fair value for the MIP units and 2015 Phantom Plan Units. We recognize expense for performance-based awards when we expect them to be earned. We recognize timed-based awards over the requisite service period, which is generally the vesting period of the award. We recognize forfeitures as they occur.
Plans”). We used independent third-party valuation specialists in 2020 and 2019 using year-to-date October data in each year to assist management in performing the annual valuation of MIP and 2015 Phantom Plan Units. We also utilized valuation specialists in April 2020 as we believed COVID-19 represented a triggering event that might indicate impairment. The specialists assistassisted management in the determination of the fair value of the awards granted using the Monte Carlo option pricing model. The subjective assumptions and the application of judgment in determining the fair value of the awards represent management’s best estimates. If factors change and different assumptions are used, our equity compensation expense could be materially different in the future. The most significant assumptions utilized in the MIP and judgments are as follows:
•Phantom Plan were expected volatility, time outstanding, risk-free rate and expected dividend yield. Expected volatility—We determine the expected price volatility was based on the historical volatilitiesvolatility of our peer group, as we do not have a sufficient trading history for our units. Industry peers consist of several public companies in the medical device industry similar to us in size, stage of life cycle and financial leverage. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own stock price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be utilized in the calculation.
•Time to liquidity event—group. The amount of time that the awards are expected to be outstanding.
•Risk-free interest rate—We based the risk-free rate was based on U.S. Government Constant Maturity Treasury rates for a term corresponding to the Timeamount of time the awards were expected to Liquidity Event.
•Expectedbe outstanding. The expected dividend yield—We used a dividendyield assumption had the rate of zero as we have not previously issued dividends and dodid not anticipate paying dividends in the foreseeable future.
Income taxes
The assumptions utilized to determine the fair value of the awards are indicated in the following table:
| | | | | | | | | | | |
| 2020 | | 2019 |
| Expected dividend yield | 0.0 | % | | 0.0 | % |
| Expected volatility | 50.0 | % | | 35.0 | % |
| Risk-free interest rate | 0.1 | % | | 1.5 | % |
| Time to exit event (in years) | 0.4 | | 1.5 |
The calculation of the fair value of awards also requirestax provision for interim periods is determined using an estimate of our equity value, based on inputs from management and reporting unit valuation reports prepared by the specialistsannual effective tax rate, adjusted for discrete items, if any, that arise during the annual goodwill impairment process.
We determined the valueperiod. Each quarter, we update our estimate of our equity utilizing methodologies, approachesannual effective tax rate, and assumptions consistent withif the American Institute of Certified Public Accountants Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation”. In the absence ofestimated annual effective tax rate changes, we make a public trading market, our Board of Managers determines a reasonablecumulative adjustment in such period. The quarterly tax provision, and estimate of the grant date fair value of our equity awards based on input from management and the annual valuation reports prepared by the specialists. In addition, we exercised judgment in evaluating and assessing the foregoing based on several factors including:
•the nature and history of our business;
•our historical operating and financial results;
•the market value of companies that are engaged in a similar business to ours;
•the lack of marketability of our common stock;
•the overall inherent risks associated with our business at the time awards were approved; and
•the overall equity market conditions and general economic trends.
As of December 31, 2020, there was approximately $9.7 million of unrecognized compensation expense to be recognized over a weighted-average period of 1.3 years.
Income taxes
BV LLC is currently a partnership for U.S. federal incomeeffective tax purposes. As a partnership, taxable income or loss is generally included in the income tax returns of its members. We also have a subsidiary that operates as a C-corporation that is subject to income tax requirements and international operations thatrate, are subject to foreignvariation due to several factors, including variability in pre-tax income (or loss), the mix of jurisdictions to which such income relates, changes in how we conduct business, and tax requirements. Additionally, BV LLClaw developments.
We maintain a valuation allowance on certain deferred tax assets that we have determined are not more-likely-than-not to be realizable and assess the need for an adjustment to this valuation allowance on a quarterly basis. The assessment is liablebased on estimates of future sources of taxable income for various other statethe jurisdictions in which we operate and local taxes. As a corporation, Bioventus Inc.the periods over which deferred tax assets will be subjectrealizable. In the event we determine we will be able to U.S. federal, state and local income taxes. realize all or part of the net deferred tax assets in the future, all or part of the valuation allowance will be reversed in the period it is determined. The release of all or part of the valuation allowance against deferred tax assets may cause greater volatility in the effective tax rate in the periods in which it is reversed.
We recognize the effect of incomea tax benefit from any uncertain tax positions only if these positionsthey are more likely than not to be sustained. We reflect changes in recognition or measurement in the period in which the change in judgment occurs. Upon the redemption or exchange of BV LLC Units for shares of Class A common stock or cash, we will determine if we are likely to realize the resulting tax benefits. If we are, we will record (i) a deferred tax assetsustained upon examination based on the step-up in basis resulting from the exchange and the then effective income tax rate, (ii) a payable to related party in respecttechnical merits of the corresponding 85% payment underposition. The amount of the TRA and (iii)accrual for which an exposure exists is measured as the largest amount of benefit determined on a tax benefit based on the net difference between (i) and (ii). As we realize cash tax savings, we will reduce the deferred tax asset and the payments made under the TRA will reduce the payable to related party. Further, we will evaluate the likelihoodcumulative probability basis that we will realize the benefit represented by the deferred tax asset and, to the extent that we estimate itbelieve is more likely than not that we will not realize the benefit, we will reduce the carrying amountto be realized upon ultimate settlement of the deferred tax asset with a valuation allowance.
Long-lived assets
Property and equipment are stated at cost and are depreciated using the straight-line method over the shorterposition. Components of the asset’s estimated useful life,reserve, if relevant, are classified as a current or noncurrent liability in the lease term ifconsolidated balance sheet based on when we expect each of the items to be settled. Interest and penalties related to leased property,unrecognized tax benefits are recognized as follows in years:a component of income tax expense.
| | | | | |
Computer software and hardware | 3-5 |
Leasehold improvements | 7 |
Machinery and equipment | 7 |
Furniture and fixtures | 7 |
We amortize finite-lived identifiable intangible assets using the straight-line method over their estimated remaining weighted average useful lives as follows in years:
| | | | | |
| Weighted
Average
Useful Life
|
Intellectual property | 17.3 |
Distribution rights | 12.7 |
Customer relationships | 10 |
Developed technology | 8.3 |
We capitalize costs incurred from third-party vendors for software design, configuration, codingEmerging Growth Company and testing and amortizes these costs on a straight-line basis over the estimated useful life of the product, not to exceed three years. We do not capitalize costs that are precluded from capitalization in authoritative guidance, such as preliminary project phase costs, planning, oversight, process re-engineering costs, training costs or data conversion costs.
The carrying values of property, equipment and finite lived intangible assets are reviewed for recoverability if the facts and circumstances suggest that a potential impairment may have occurred. If this review indicates that carrying values may not be recoverable, we will perform an assessment to determine if an impairment charge is required to reduce carrying values to estimated fair value. There were no events, facts or circumstances for the years ended December 31, 2020 and 2019 that resulted in any impairment charges to our property, equipment or finite lived intangible assets.
JOBS ActSmaller Reporting Company Status
We qualify as an “emerging growth company” pursuant to the provisions of the JOBS Act. For as long as we are an “emerging growth company,” we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, reduced disclosure obligations relating to the presentation of financial statements in the “Management’s discussion and analysis of financial condition and results of operations” section and exemptions from the requirements of holding advisory “say-on-pay” votes on executive compensation and shareholder advisory votes on golden parachute compensation. We have availed ourselves of the reduced reporting obligations and executive compensation disclosure in this Annual Report and expect to continue to avail ourselves of the reduced reporting obligations available to emerging growth companies in future filings.
In addition, an emerging growth company can delay its adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we plan to comply with any new or revised accounting standards on the relevant dates on which non- emergingnon-emerging growth companies must adopt such standards. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
We will continue to qualify as an emerging growth company until the earliest of:
•The last day of our fiscal year following the fifth anniversary of the date of our IPO;
•The last day of our fiscal year in which have annual gross revenues of $1.07$1.235 billion or more;
•The date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt;
•The date on which we are deemed to be a “large accelerated filer”, which will occur at such time as we (1) have an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of our most recently completed second fiscal quarter, (2) have been required to file annual and quarterly reports under the Exchange Act for a period of at least 12 months, and (3) have filed at least one annual report pursuant to the Exchange Act.Act, and (4) are not eligible to use the requirements for smaller reporting companies as defined in Rule 12b-2 under the Exchange Act (annual revenue less than $100 million and either no public float or a public float of less than $700 million).
Additionally, we are considered a “smaller reporting company,” as defined by Rule 12b-2 of the Exchange Act, which was determined as of the last day of our second fiscal quarter of 2023 (a “determination date”). We will continue to be categorized as a smaller reporting company—accelerated filer until our public float reaches $250 million at a future determination date.
If we are a smaller reporting company at the time we cease to be an emerging growth company, we may rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations including regarding executive compensation.
Item 7A.Quantitative and Qualitative Disclosures Aboutabout Market Risk.
Quantitative and qualitative disclosures about market risk
We are exposed to various market risks, which may result in potential losses arising from adverse changes in market rates, such as interest rates and foreign exchange rates. We do not enter into derivatives or other financial instruments for trading or speculative purposes. We use derivative instruments to manage exposures to interest rates and foreign currencies. Derivatives are recorded on the balance sheet at fair value at each balance sheet date. We have elected the fair value method of accounting and do not designate whether the derivative instrument is an effective hedge of an asset, liability or firm commitment. Changes in the fair values of derivative instruments are recognized in the consolidated statements of operations and comprehensive (loss) income (loss) in the period incurred.
Interest rate risk
Our cash and cash equivalents balance as of December 31, 20202023 consisted of demand deposits and institutional money market funds held in U.S. and foreign banks. Cash equivalents consist of highly liquid investment securities with original maturities on the date of purchase of three months or less and can be exchanged for a known amount of cash. We are exposed to the market risk related to fluctuations in interest rates and market prices for our cash equivalents. We are also exposed to interest rate risk in connection with borrowings under our credit agreement,Amended 2019 Credit Agreement, which bear interest at a floating rate based on one-month LIBORSOFR plus an applicable borrowing margin. As of December 31, 2020,2023, a 1.0% increase in interest rate would result in $8.4$9.7 million increase in total interest payable over the remaining life of the credit agreementAmended 2019 Credit Agreement in the event we were to draw down the entire capacity of our revolving credit facility. For variable rate debt, interest rate changes generally do not affect the fair value of the debt instrument,Amended 2019 Credit Agreement, but impact future earnings and cash flows, assuming other factors are constant. In the ordinary course of business, we may enter into contractual arrangements to reduce our exposure to interest rate risks.risks, subject to any applicable limitations in our financing arrangements.
In March 2020, we entered intoWe had an interest rate swap agreement effective March 26, 2020 and expiring December 6, 2024 to limit our exposure to changes in the variable interest rate on our term loan. The derivative instrument was not designated as a hedge. We terminated this interest rate swap agreement on October 28, 2022.
Foreign exchange risk management
We operate in countries other than the U.S.United States and are exposed to foreign currency risks. We bill most direct sales outside of the U.S.United States in local currencies. We expect that the percentage of our sales denominated in foreign currencies will increase in the foreseeable future as we continue to expand into international markets. When sales or expenses are not denominated in U.S. dollars, a fluctuation in exchange rates could affect our net income. We believe that the risk of a significant impact on our operating income from foreign currency fluctuations is minimal. Although we do not currently have any foreign currency hedges, we have used foreign exchange forward contracts in the past to protect against the impact of foreign currency fluctuations and may use forward contracts, derivatives or other hedges for foreign exchange risk management purposes in the future.future, subject to any applicable limitations in our financing arrangements.
Effects of inflation
We do not believe that inflation has had a material effect on our results of operations during the periods presented herein.
Item 8.Financial Statements and Supplementary Data.
Index to Consolidated Financial Statements
| | | | | |
| Page |
| Page |
| Bioventus Inc. | |
| |
| |
| |
| |
| |
Bioventus LLC | |
| |
| |
| |
| |
| |
| |
| | | | | | | | |
GRANT THORNTON LLP | | REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
4140 ParkLake Ave., Suite 130 | | |
Raleigh, NC 27612-3723 | | |
| | |
D +1 919 881 2700 | | |
F +1 919 881 2795 | | |
Board of Directors and Shareholders
Bioventus Inc.
Opinion on the financial statements
We have audited the accompanying balance sheets of Bioventus Inc. (a Delaware corporation) (the “Company”) as of December 31, 2020 and 2019 and the related notes (collectively referred to as the “financial statement”). In our opinion, the financial statement presents fairly, in all material respects, the financial position of the Companyas of December 31, 2020 and 2019, in conformity with accounting principles generally accepted in the United States of America.
Basis for opinion
This financial statement is the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statement based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statement is free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statement, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statement. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statement. We believe that our audits provide a reasonable basis for our opinion.
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2019.
Raleigh, North Carolina
March 26, 2021
BIOVENTUS INC.
Balance sheets
December 31, 2020 and 2019
(Amounts in thousands, except share and per share data)
| | | | | | | | | | | |
| 2020 | | 2019 |
| Assets | $ | — | | | $ | — | |
| Liabilities | — | | | — | |
| Commitments and contingencies | | | |
| Stockholders’ equity | — | | | — | |
| Common stock, $0.01 par value - 10 shares authorized, issued and outstanding | — | | | — | |
| Total liabilities and stockholders’ equity | $ | — | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements.
BIOVENTUS INC.
Notes to balance sheets
(Amounts in thousands, except share and per share amounts)
1. Organization
The Company
Bioventus Inc. (the Company, we, us or our) was formed as a Delaware corporation on December 22, 2015 for the purpose of facilitating an initial public offering (IPO) and other related transactions in order to carry on the business of Bioventus LLC and its subsidiaries (BV LLC). The notes to these financial statements should be read in conjunction with notes to BV LLC’s financial statements. As of December 31, 2020, the Company had not engaged in any business activities except in connection with its formation. On February 16, 2021, the Company closed an IPO of 9,200,000 shares of Class A common stock at a public offering price of $13.00 per share, which includes 1,200,000 shares issued pursuant to the underwriters' over-allotment option. The Company received $111,228 in proceeds, net of underwriting discounts and commissions of $8,372, which was used to purchase newly-issued membership interests from BV LLC at a price per interest equal to the IPO price of $13.00. In addition to the underwriting discounts and commission the Company incurred offering expenses totaling $4,310. Subsequent to the IPO and related transactions that occurred in connection with the IPO (the Transactions) as described in Note 4, the Company is the sole managing member of BV LLC and owns 72.2% of BV LLC. The Company has a majority economic interest, the sole voting interest in, and controls the management of BV LLC. As a result, subsequent to the IPO, the Company will consolidate the financial results of BV LLC and will report a non-controlling interest representing the 27.8% interest not held by the Company.
2. Summary of significant accounting policies
Basis of accounting
The balance sheets have been prepared in accordance with accounting principles generally accepted in the United States of America. Separate statements of income, comprehensive income, changes in stockholder's equity, and cash flows have not been presented in the financial statements as there has been no activity.
3. Stockholder's equity
The Corporation had 10 outstanding shares at a nominal value as of December 31, 2020.
4. Subsequent event
Transactions
The Company and BV LLC completed the following Transactions regarding the IPO.
BV LLC amended and restated the Bioventus LLC Agreement, to, among other things, (i) provide for a new single class of common membership interests in BV LLC (LLC Interests), (ii) exchange all of the existing membership interests in BV LLC for new LLC Interests and (iii) appoint Bioventus Inc. as the sole managing member of BV LLC.
The Company amended and restated its certificate of incorporation to, among other things, provide for the (i) authorization of 250,000,000 shares of Class A common stock with a par value of $0.001 per share; (ii) authorization of 50,000,000 shares of Class B common stock with a par value of $0.001 per share; (iii) authorization of 10,000,000 shares of undesignated preferred stock that may be issued from time to time by the Company's Board of Directors (BOD) in one or more series; and (iv) establishment of a classified BOD, divided into three classes, each of whose members will serve for staggered three-year terms.
Holders of Class A and Class B common stock are entitled to one vote per share and, except as otherwise required, will vote together as a single class on all matters on which stockholders generally are entitled to vote. Holders of Class B common stock are not entitled to receive dividends and will not be entitled to receive any distributions upon the liquidation, dissolution or winding up of the Company. Shares of Class B common stock may only be issued to the extent necessary to maintain the one-to-one ratio between the number of LLC Interests held by the only member of BV LLC that remained a member (Continuing LLC Owner) and the number of shares of Class B common stock held by the Continuing LLC Owner. Shares of Class B common stock are transferable only together with an equal number of LLC Interests. Shares of Class B common stock will be canceled on a one-for-one basis if the Company, at the election of a Continuing LLC Owner, redeem or exchange LLC Interests.
The Company’s amended and restated certificate of incorporation and the Bioventus LLC Agreement requires that the Company and BV LLC at all times maintain a one-to-one ratio between the number of shares of Class A common stock issued by the Company and the number of LLC Interests owned by the Company, as well as a one-to-one ratio between the number of shares of Class B common stock owned by the Continuing LLC Owner and the number of LLC Interests owned by the Continuing LLC Owner.
The Company acquired, by merger, ten entities that were members of BV LLC (Former LLC Owners), for which the Company issued 31,838,589 shares of Class A common stock as merger consideration (Merger). The only assets held by the Former LLC Owners were 31,838,589 LLC Interests and a corresponding number of shares of Class B common stock. Upon consummation of the Merger, the Company canceled the 31,838,589 shares of Class B common stock and recognized the 31,838.589 of LLC Interests at carrying value, as the Merger is considered to be a transaction between entities under common control. Following the Merger and IPO, the Company now holds 41,038,589 LLC Interests, representing a 72.2% ownership interest in BV LLC.
Tax Receivable Agreement
The Company expects to obtain an increase in the share of the tax basis of the assets of BV LLC when LLC Interests are redeemed or exchanged by the Continuing LLC Owner and other qualifying transactions. This increase in tax basis may have the effect of reducing the amounts that the Company would otherwise pay in the future to various tax authorities. The increase in tax basis may also decrease gains (or increase losses) on future dispositions of certain capital assets to the extent tax basis is allocated to those capital assets.
On February 16, 2021, the Company entered into a tax receivable agreement (TRA) with the Continuing LLC Owner that provides for the payment by the Company to the Continuing LLC Owner of 85% of the amount of tax benefits, if any, that Bioventus actually realizes as a result of (i) increases in the tax basis of assets of BV LLC resulting from any redemptions or exchanges of LLC Interests or any prior sales of interests in BV LLC and (ii) certain other tax benefits related to our making payments under the TRA.
Equity-Based Compensation
In February 2021, in connection with the IPO, the BV LLC Phantom Profits Interest Plan (Phantom Plan) terminated and the Company assumed the obligations of settling the vested awards. The awards will be settled 12 months following the Phantom Plan termination. Vested awardees whose BV LLC employment terminated prior to the IPO will have their awards settled in cash totaling $10,875. Vested awardees that were active BV LLC employees at the IPO will receive 798,422 shares of Class A common stock.
In February 2021, in connection with the IPO, the Company began operating two equity-based compensation plans. These are the Bioventus Inc. 2021 Incentive Award Plan (2021 Plan) and the Bioventus Inc. 2021 Employee Stock Purchase Plan (ESPP).
2021 Plan
The 2021 Plan is designed to grant incentive awards to eligible employees and other service providers in order to attract, motivate and retain the talent for which the Company competes. The 2021 Plan provides for the grant of stock options, including incentive stock options and nonqualified stock options, restricted stock, dividend equivalents, restricted stock units (RSUs), other stock-based awards, and cash awards.
In conjunction with the IPO, 7,592,476 shares of Class A common stock were authorized for issuance. The number of shares available for issuance will be increased by an annual increase on January 1 of each calendar year beginning in 2022 and ending in and including 2031, equal to the lesser of (i) 4.5% of the shares of our Class A common stock outstanding on the final day of the immediately preceding calendar year and (ii) a smaller number of shares as determined by our board of directors.
In conjunction with the IPO, 4,561,500 stock options were granted to certain employees with an exercise price of $13.00 per share and vest equally over two or four years as indicated in each option agreement. Additionally, the Company granted 360,670 RSUs to the directors and certain employees which vest equally over one or four years as indicated in each RSU agreement and entitles the recipient to the same number of shares of Class A common stock. 2,670,306 shares of Class A common stock remain available for issuance under the 2021 Plan.
ESPP
The ESPP allows for the Company to grant options to employees to purchase shares of the Company’s Class A common stock through payroll deductions of up to 15% of their eligible compensation as defined in the ESPP.
In conjunction with the IPO, 542,320 shares of Class A common stock were authorized for issuance. The number of shares available for issuance will be increased by an annual increase on January 1 of each calendar year beginning in 2022 and ending in and including 2031, equal to the lesser of (i) 1% of the shares of our Class A common stock outstanding on the final day of the immediately preceding calendar year and (ii) a smaller number of shares as determined by our board of directors. There have been no grants under the ESPP as of March 22, 2021.
| | | | | | | | |
GRANT THORNTON LLP | | REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
4140 ParkLake Ave., Suite 130 | | |
Raleigh, NC 27612-3723 | | |
| | |
D +1 919 881 2700 | | |
F +1 919 881 2795 | | |
Board of Directors and Shareholders
Bioventus Inc.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Bioventus LLCInc. (a Delaware limited liability company)corporation) and subsidiaries (the “Company”) as of December 31, 20202023 and 2019,2022, the related consolidated statements of operations and comprehensive (loss) income, (loss), changes in stockholders’ and members’ equity, and cash flows for each of the three years thenin the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Companyas of December 31, 20202023 and 2019,2022, and the results of itsoperations and itscash flows for each of the three years thenin the period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2019.
Raleigh, North Carolina
March 26, 2021
12, 2024
Report of Independent Registered Public Accounting Firm
To the Board of Managers and Members of Bioventus LLC
Opinion on the Financial Statements
We have audited the consolidated statements of operations and comprehensive income (loss), of changes in members’ equity and of cash flows of Bioventus LLC and its subsidiaries (the “Company”) for the year ended December 31, 2018, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the results of operations and cash flows of the Company for the year ended December 31, 2018 in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit of these consolidated financial statements in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
Emphasis of Matter
As discussed in Notes 2 and 12 to the consolidated financial statements, the Company has identified noncompliance with certain U.S. Federal statutes and regulations to which the Company is subject and made a voluntary self-disclosure to the U.S. Department of Health and Human Services Office of Inspector General. As a result of the noncompliance, the Company may be subject to civil monetary penalties, as well as repayment obligations related to previously reimbursed claims and fines. Management’s evaluation of the impact of these material contingencies is also discussed in Note 12.
/s/ PricewaterhouseCoopers LLP
Raleigh, North Carolina
August 16, 2019, except for the effects of disclosing net loss per unit information discussed in Note 13 and the effects of discontinued operations discussed in Note 16 to the consolidated financial statements, as to which the date is October 6, 2020
We served as the Company's auditor from 2012 to 2019.
BIOVENTUS LLCBioventus Inc.
Consolidated statementsStatements of operationsOperations and comprehensive income (loss)Comprehensive (Loss) Income
Years endedEnded December 31, 2020, 20192023, 2022 and 20182021
(Amounts in thousands, except unit and per unit data)share amounts)
| | | | | | | | | | | | | | | | | |
| | 2020 | | 2019 | | 2018 |
| Net sales | $ | 321,161 | | | $ | 340,141 | | | $ | 319,177 | |
Cost of sales (including depreciation and amortization
of $21,169, $22,399 and $20,614, respectively) | 87,642 | | | 90,935 | | | 84,168 | |
| Gross profit | 233,519 | | | 249,206 | | | 235,009 | |
| Selling, general and administrative expense | 193,078 | | | 198,475 | | | 191,672 | |
| Research and development expense | 11,202 | | | 11,055 | | | 8,095 | |
| Change in fair value of contingent consideration | — | | | — | | | (739) | |
| Restructuring costs | 563 | | | 575 | | | 1,373 | |
| Depreciation and amortization | 7,439 | | | 7,908 | | | 8,615 | |
| Loss on impairment of intangible assets | — | | | — | | | 489 | |
| Operating income | 21,237 | | | 31,193 | | | 25,504 | |
| Interest expense | 9,751 | | | 21,579 | | | 19,171 | |
| Other (income) loss | (4,428) | | | (75) | | | 226 | |
| Other expense | 5,323 | | | 21,504 | | | 19,397 | |
| Income from continuing operations before income taxes | 15,914 | | | 9,689 | | | 6,107 | |
| Income tax expense | 1,192 | | | 1,576 | | | 1,664 | |
| Net income from continuing operations | 14,722 | | | 8,113 | | | 4,443 | |
| Loss from discontinued operations, net of tax | — | | | 1,815 | | | 16,650 | |
| Net income (loss) | 14,722 | | | 6,298 | | | (12,207) | |
| Loss attributable to noncontrolling interest | 1,689 | | | 553 | | | — | |
| Net income (loss) attributable to unit holders | 16,411 | | | 6,851 | | | (12,207) | |
| Other comprehensive income (loss), net of tax | | | | | |
Change in prior service cost and unrecognized
(loss) gain for defined benefit plan adjustment | (54) | | | (78) | | | 131 | |
| Change in foreign currency translation adjustments | 2,126 | | | (322) | | | (334) | |
| Other comprehensive income (loss) | 2,072 | | | (400) | | | (203) | |
| Comprehensive income (loss) | $ | 18,483 | | | $ | 6,451 | | | $ | (12,410) | |
| | | | | | |
| Net income from continuing operations attributable to unit holders | $ | 16,411 | | | $ | 8,666 | | | $ | 4,443 | |
| Accumulated and unpaid preferred distributions | (6,133) | | | (5,955) | | | (5,781) | |
| Net income allocated to participating shareholders | (5,895) | | | (1,555) | | | — | |
Net income (loss) from continuing operations attributable to
common unit holders | 4,383 | | | 1,156 | | | (1,338) | |
| Loss from discontinued operations, net of tax | — | | | 1,815 | | | 16,650 | |
| Net income (loss) attributable to common unit holders | $ | 4,383 | | | $ | (659) | | | $ | (17,988) | |
| Net income (loss) per unit attributable to common unit holders - basic and diluted | | | | | |
| Net income (loss) from continuing operations | $ | 0.89 | | | $ | 0.24 | | | $ | (0.27) | |
| Loss from discontinued operations, net of tax | — | | | 0.37 | | | 3.40 | |
| Net income (loss) attributable to common unit holders | $ | 0.89 | | | $ | (0.13) | | | $ | (3.67) | |
| | | | | |
Weighted average units used in computing basic and
diluted net income (loss) per common unit | 4,900,000 | | | 4,900,000 | | | 4,900,000 | |
| | | | | | | | | | | | | | | | | |
| 2023 | | 2022 | | 2021 |
| Net sales | $ | 512,345 | | | $ | 512,117 | | | $ | 430,898 | |
Cost of sales (including depreciation and amortization of $48,503, $45,622 and $26,471 respectively) | 184,152 | | | 181,037 | | | 128,192 | |
| Gross profit | 328,193 | | | 331,080 | | | 302,706 | |
| Selling, general and administrative expense | 303,879 | | | 332,134 | | | 254,253 | |
| Research and development expense | 13,446 | | | 23,854 | | | 19,039 | |
| Restructuring costs | 840 | | | 6,779 | | | 2,487 | |
| Change in fair value of contingent consideration | 719 | | | 1,102 | | | 829 | |
| Depreciation and amortization | 8,842 | | | 9,748 | | | 8,363 | |
| Impairment of assets | 78,615 | | | — | | | — | |
| Impairment of goodwill | — | | | 124,697 | | | — | |
| Loss on disposals | 3,577 | | | — | | | — | |
| Impairment of variable interest entity assets | — | | | — | | | 5,674 | |
| Operating (loss) income | (81,725) | | | (167,234) | | | 12,061 | |
| Interest expense, net | 40,676 | | | 12,021 | | | 1,112 | |
| Other (income) expense | (1,290) | | | 9,770 | | | 1,461 | |
| Other expense | 39,386 | | | 21,791 | | | 2,573 | |
| (Loss) income before income taxes | (121,111) | | | (189,025) | | | 9,488 | |
| Income tax expense (benefit), net | 85 | | | (44,374) | | | (1,966) | |
| Net (loss) income from continuing operations | (121,196) | | | (144,651) | | | 11,454 | |
| Loss from discontinued operations, net of tax | (74,429) | | | (68,740) | | | (1,868) | |
| Net (loss) income | (195,625) | | | (213,391) | | | 9,586 | |
| Loss attributable to noncontrolling interest - continuing operations | 24,458 | | | 40,732 | | | 9,789 | |
| Loss attributable to noncontrolling interest - discontinued operations | 14,937 | | | 13,955 | | | — | |
| Net (loss) income attributable to Bioventus Inc. | $ | (156,230) | | | $ | (158,704) | | | $ | 19,375 | |
| | | | | |
| Net (loss) income | $ | (195,625) | | | $ | (213,391) | | | $ | 9,586 | |
| Other comprehensive (loss) income, net of tax | | | | | |
Change in prior service cost and unrecognized (loss) gain
for defined benefit plan adjustment | (8) | | | 133 | | | 60 | |
| Change in foreign currency translation adjustments | 1,140 | | | (501) | | | (1,318) | |
| Comprehensive (loss) income | (194,493) | | | (213,759) | | | 8,328 | |
| Comprehensive loss attributable to noncontrolling interest - continuing operations | 24,230 | | | 40,811 | | | 9,789 | |
| Comprehensive loss attributable to noncontrolling interest - discontinued operations | 14,937 | | | 13,955 | | | — | |
| Comprehensive (loss) income attributable to Bioventus Inc. | $ | (155,326) | | | $ | (158,993) | | | $ | 18,117 | |
| | | | | |
Loss per share of Class A common stock from(1): | | | | | |
| Continuing operations, basic and diluted | $ | (1.54) | | | $ | (1.70) | | | $ | (0.11) | |
| Discontinued operations, basic and diluted | (0.95) | | | (0.89) | | | (0.04) | |
| Loss per share of Class A common stock, basic and diluted | $ | (2.49) | | | $ | (2.59) | | | $ | (0.15) | |
| | | | | |
Weighted-average shares of Class A common stock outstanding, basic and diluted(1): | 62,647,554 | | 61,389,107 | | 45,472,483 | |
(1) Per share information for the year ended December 31, 2021 represents loss per share of Class A common stock and weighted-average shares of Class A common stock outstanding from February 16, 2021 through December 31, 2021, the period following Bioventus Inc.'s initial public offering and related transactions described in Note 8. Stockholders’ equity and Note 9. Earnings per share within the Notes to the Consolidated Financial Statements. |
The accompanying notes are an integral part of these consolidated financial statements.
BIOVENTUS LLCBioventus Inc.
Consolidated balance sheets
Balance Sheets as of December 31, 20202023 and 20192022
(Amounts in thousands)thousands, except share amounts)
| | 2020 | | 2019 |
| 2023 | | | 2023 | | 2022 |
| Assets | Assets | | | |
| Current assets: | Current assets: | |
| Current assets: | |
| Current assets: | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Cash and cash equivalents | Cash and cash equivalents | $ | 86,839 | | | $ | 64,520 | |
| Accounts receivable, net | Accounts receivable, net | 88,283 | | | 85,128 | |
| Inventory | Inventory | 29,120 | | | 27,326 | |
| Prepaid and other current assets | Prepaid and other current assets | 7,552 | | | 6,059 | |
| Current assets attributable to discontinued operations | |
| Total current assets | Total current assets | 211,794 | | | 183,033 | |
| | Property and equipment, net | |
| Property and equipment, net | |
| Property and equipment, net | Property and equipment, net | 6,879 | | | 4,489 | |
| Goodwill | Goodwill | 49,800 | | | 49,800 | |
| Intangible assets, net | Intangible assets, net | 191,650 | | | 216,510 | |
| Operating lease assets | Operating lease assets | 14,961 | | | 15,267 | |
| Investments and other assets | 19,382 | | | 3,308 | |
| Investment and other assets | |
| Long-term assets attributable to discontinued operations | |
| Total assets | Total assets | $ | 494,466 | | | $ | 472,407 | |
| | Liabilities and Members’ Equity | |
| Liabilities and Stockholders' Equity | |
| Liabilities and Stockholders' Equity | |
| Liabilities and Stockholders' Equity | |
| Current liabilities: | |
| Current liabilities: | |
| Current liabilities: | Current liabilities: | |
| Accounts payable | Accounts payable | $ | 4,422 | | | $ | 6,440 | |
| Accounts payable | |
| Accounts payable | |
| Accrued liabilities | Accrued liabilities | 88,187 | | | 52,827 | |
| Accrued equity-based compensation | 11,054 | | | 15,547 | |
| Current portion of long-term debt | Current portion of long-term debt | 15,000 | | | 10,000 | |
| Other current liabilities | Other current liabilities | 3,926 | | | 4,201 | |
| Current liabilities attributable to discontinued operations | |
| Total current liabilities | Total current liabilities | 122,589 | | | 89,015 | |
| | Long-term debt, less current portion | Long-term debt, less current portion | 173,378 | | | 187,965 | |
| Accrued equity-based compensation, less current portion | 29,249 | | | 25,255 | |
| Deferred tax liability | 3,362 | | | 3,874 | |
| Long-term debt, less current portion | |
| Long-term debt, less current portion | |
| Deferred income taxes | |
| Contingent consideration | |
| Other long-term liabilities | Other long-term liabilities | 21,728 | | | 20,681 | |
| Long-term liabilities attributable to discontinued operations | |
| Total liabilities | Total liabilities | 350,306 | | | 326,790 | |
| | Commitments and contingencies (Note 12) | Commitments and contingencies (Note 12) | |
| Commitments and contingencies (Note 12) | |
| Commitments and contingencies (Note 12) | | | | |
| Members’ equity (preferred unit liquidation preference of $210,576
and $204,443 at December 31, 2020 and 2019, respectively) | 285,173 | | | 285,147 | |
| Stockholders’ Equity: | |
| Stockholders’ Equity: | |
| Stockholders’ Equity: | |
| Preferred stock, $0.001 par value, 10,000,000 shares authorized, 0 shares issued | |
| Preferred stock, $0.001 par value, 10,000,000 shares authorized, 0 shares issued | |
| Preferred stock, $0.001 par value, 10,000,000 shares authorized, 0 shares issued | |
Class A common stock, $0.001 par value, 250,000,000 shares authorized as of December 31, 2023 and December 31, 2022, 63,267,436 and 62,063,014 shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | |
Class A common stock, $0.001 par value, 250,000,000 shares authorized as of December 31, 2023 and December 31, 2022, 63,267,436 and 62,063,014 shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | |
Class A common stock, $0.001 par value, 250,000,000 shares authorized as of December 31, 2023 and December 31, 2022, 63,267,436 and 62,063,014 shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | |
Class B common stock, $0.001 par value, 50,000,000 shares authorized, 15,786,737 shares issued and outstanding as of December 31, 2023 and December 31, 2022 | |
| Additional paid-in capital | |
| Accumulated deficit | |
| Accumulated other comprehensive income (loss) | Accumulated other comprehensive income (loss) | 1,607 | | | (465) | |
| Accumulated deficit | (144,539) | | | (141,700) | |
| Equity attributable to unit holders | 142,241 | | | 142,982 | |
| Total stockholders’ equity attributable to Bioventus Inc. | |
| Noncontrolling interest | Noncontrolling interest | 1,919 | | | 2,635 | |
| Total members’ equity | 144,160 | | | 145,617 | |
| Total liabilities and members’ equity | $ | 494,466 | | | $ | 472,407 | |
| Total stockholders’ equity | |
| Total liabilities and stockholders’ equity | |
The accompanying notes are an integral part of these consolidated financial statements.
BIOVENTUS LLCBioventus Inc.
Consolidated statementsStatements of changesChanges in members’ equityStockholders’ and Members’ Equity
Years endedEnded December 31, 2020, 20192023, 2022 and 20182021
(Amounts in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Members’
Equity | | Class A Common Stock | | Class B Common Stock | | Additional Paid-in Capital | | Accumulated other comprehensive income (loss) | | Accumulated Deficit | | Non-
controlling
Interest | | Total Stockholders' and
Members’
Equity |
| | Shares | Amount | | Shares | Amount | | | | | |
| December 31, 2020 | $ | 144,160 | | | — | | $ | — | | | — | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 144,160 | |
| Prior to Organizational Transactions: |
| Refund from members | 123 | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | 123 | |
| Equity-based compensation | (39) | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (39) | |
| Net income | 25,977 | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | 25,977 | |
| Other comprehensive loss | (1,507) | | | — | | — | | | — | | — | | | — | | | — | | | — | | | — | | | (1,507) | |
| Effect of Organizational Transactions | (168,714) | | | 31,838,589 | | 32 | | | 15,786,737 | | 16 | | | 41,813 | | | — | | | — | | | 79,119 | | | (47,734) | |
| Subsequent to Organizational Transactions: |
| Initial public offering, net of offering costs | — | | | 9,200,000 | | 9 | | | — | | — | | | 106,441 | | | — | | | — | | | — | | | 106,450 | |
| Issuance of Class A common stock for equity plans | — | | | 169,125 | | — | | | — | | — | | | 1,617 | | | — | | | — | | | — | | | 1,617 | |
| Issuance of Class A common stock for acquisitions, net of registration fees | — | | | 18,340,790 | | 18 | | | — | | — | | | 272,706 | | | — | | | — | | | — | | | 272,724 | |
| Distribution to Continuing LLC Owner | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (3,306) | | | (3,306) | |
| Net loss | — | | | — | | — | | | — | | — | | | — | | | — | | | (6,602) | | | (9,789) | | | (16,391) | |
| Deconsolidation of variable interest entity | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 3,746 | | | 3,746 | |
| Equity based compensation | — | | | — | | — | | | — | | — | | | 15,059 | | | — | | | — | | | 4,825 | | | 19,884 | |
| Replacement equity awards in connection with acquisitions | — | | | — | | — | | | — | | — | | | 27,636 | | | — | | | — | | | — | | | 27,636 | |
| Acquisition of noncontrolling interest | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 200 | | | 200 | |
| Change in noncontrolling interest allocations | — | | | — | | — | | | — | | — | | | 8,046 | | | — | | | — | | | 65,821 | | | 73,867 | |
| Other comprehensive income | — | | | — | | — | | | — | | — | | | — | | | 179 | | | — | | | 70 | | | 249 | |
| December 31, 2021 | $ | — | | | 59,548,504 | $ | 59 | | | 15,786,737 | $ | 16 | | | $ | 473,318 | | | $ | 179 | | | $ | (6,602) | | | $ | 140,686 | | | $ | 607,656 | |
| Issuance of Class A common stock for equity plans | | | 2,514,510 | | 3 | | | — | | — | | | 5,819 | | | — | | | — | | | — | | 5,822 | |
| Deferred taxes on equity rebalancing | | | — | | — | | | — | | — | | | (1,977) | | | — | | | — | | | — | | | (1,977) | |
| Net loss | | | — | | — | | | — | | — | | | — | | | — | | | (158,704) | | | (54,687) | | | (213,391) | |
| Tax withholdings on equity compensation awards | | | — | | — | | | — | | — | | | (3,352) | | | — | | | — | | | — | | | (3,352) | |
| Deconsolidation of noncontrolling interest | | | — | | — | | | — | | — | | | — | | | — | | | — | | | 247 | | | 247 | |
| Equity based compensation | | | — | | — | | | — | | — | | | 14,180 | | | — | | | — | | | 3,405 | | | 17,585 | |
| Change in noncontrolling interest allocations | | | — | | — | | | — | | — | | | 2,588 | | | — | | | — | | | (2,588) | | | — | |
| Other comprehensive loss | | | — | | — | | | — | | — | | | — | | | (289) | | | — | | | (79) | | | (368) | |
| December 31, 2022 | | | 62,063,014 | | $ | 62 | | | 15,786,737 | | $ | 16 | | | $ | 490,576 | | | $ | (110) | | | $ | (165,306) | | | $ | 86,984 | | | $ | 412,222 | |
| Issuance of Class A common stock for equity plans | | | 1,204,422 | | 1 | | | — | | — | | | 777 | | | — | | | — | | | — | | | 778 | |
| Net loss | | | — | | — | | | — | | — | | | — | | | — | | | (156,230) | | | (39,395) | | | (195,625) | |
| Equity based compensation | | | — | | — | | | — | | — | | | 2,388 | | | — | | | — | | | 334 | | | 2,722 | |
| Change in noncontrolling interest allocations | | | — | | — | | | — | | — | | | 513 | | | — | | | — | | | (513) | | | — | |
| Distributions to members | | | — | | — | | | — | | — | | | — | | | — | | | — | | | (111) | | | (111) | |
| Other comprehensive income | | | — | | — | | | — | | — | | | — | | | 904 | | | — | | | 228 | | | 1,132 | |
| December 31, 2023 | | | 63,267,436 | | $ | 63 | | | 15,786,737 | | $ | 16 | | | $ | 494,254 | | | $ | 794 | | | $ | (321,536) | | | $ | 47,527 | | | $ | 221,118 | |
The accompanying notes are an integral part of these consolidated financial statements.
Bioventus Inc.
Consolidated Statements of Cash Flows
Years Ended December 31, 2023, 2022 and 2021
(Amounts in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Members’ Equity | | Accumulated Other Comprehensive Income (Loss) | | Accumulated Deficit | | Noncontrolling Interest | | Total Members’ Equity |
| Balance at December 31, 2017 | $ | 285,114 | | | $ | 138 | | | $ | (119,795) | | | $ | — | | | $ | 165,457 | |
| Profits interest compensation | 39 | | | — | | | — | | | — | | | 39 | |
| Distribution to members | — | | | — | | | (7,819) | | | — | | | (7,819) | |
| Net loss | — | | | — | | | (12,207) | | | — | | | (12,207) | |
| Defined benefit plan adjustment | — | | | 131 | | | — | | | — | | | 131 | |
| Translation adjustment | — | | | (334) | | | — | | | — | | | (334) | |
| Balance at December 31, 2018 | 285,153 | | | (65) | | | (139,821) | | | — | | | 145,267 | |
| Profits interest forfeitures | (6) | | | — | | | — | | | — | | | (6) | |
| Distribution to members | — | | | — | | | (8,730) | | | — | | | (8,730) | |
| Acquisition of noncontrolling interest | — | | | — | | | — | | | 3,188 | | | 3,188 | |
| Net income (loss) | — | | | — | | | 6,851 | | | (553) | | | 6,298 | |
| Defined benefit plan adjustment | — | | | (78) | | | — | | | — | | | (78) | |
| Translation adjustment | — | | | (322) | | | — | | | — | | | (322) | |
| Balance at December 31, 2019 | 285,147 | | | (465) | | | (141,700) | | | 2,635 | | | 145,617 | |
| Profits interest forfeitures | (12) | | | — | | | — | | | — | | | (12) | |
| Distribution to members | — | | | — | | | (19,250) | | | — | | | (19,250) | |
| Other equity compensation | 38 | | | — | | | — | | | — | | | 38 | |
| Debt conversion | — | | | — | | | — | | | 973 | | | 973 | |
| Net income (loss) | — | | | — | | | 16,411 | | | (1,689) | | | 14,722 | |
| Defined benefit plan adjustment | — | | | (54) | | | — | | | — | | | (54) | |
| Translation adjustment | — | | | 2,126 | | | — | | | — | | | 2,126 | |
| Balance at December 31, 2020 | $ | 285,173 | | | $ | 1,607 | | | $ | (144,539) | | | $ | 1,919 | | | $ | 144,160 | |
| | | | | | | | | | | | | | | | | |
| 2023 | | 2022 | | 2021 |
| Operating activities: | | | | | |
| Net (loss) income | $ | (195,625) | | | $ | (213,391) | | | $ | 9,586 | |
| Less: Loss from discontinued operations, net of tax | (74,429) | | | (68,740) | | | (1,868) | |
| (Loss) income from continuing operations | (121,196) | | | (144,651) | | | 11,454 | |
| Adjustments to reconcile net (loss) income to net cash from operating activities: | | | | | |
| Depreciation and amortization | 57,365 | | | 55,398 | | | 34,875 | |
| (Benefit) provision for expected credit losses | (1,103) | | | 5,190 | | | 485 | |
| Equity-based compensation from 2021 Stock Incentive Plan | 2,722 | | | 17,585 | | | 19,844 | |
| Profits interest plan, liability-classified and other equity awards compensation | — | | | — | | | (24,356) | |
| Change in fair value of contingent consideration | 719 | | | 1,102 | | | 829 | |
| Change in fair value of interest rate swap | — | | | (6,396) | | | (2,730) | |
| Deferred income taxes | (2,377) | | | (46,658) | | | (9,756) | |
| Change in fair value of Equity Participation Rights | — | | | — | | | (2,774) | |
| Impairments of assets | 78,615 | | | 134,982 | | | 7,043 | |
| Loss on debt retirement and modification | — | | | — | | | 2,162 | |
| Loss on disposals | 3,577 | | | — | | | — | |
| Unrealized loss (gain) on foreign currency fluctuations | 665 | | | 1,383 | | | 472 | |
| Other, net | 1,707 | | | 388 | | | 588 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable | 10,055 | | | (17,672) | | | (20,052) | |
| Inventory | (5,991) | | | (18,618) | | | 3,183 | |
| Accounts payable and accrued expenses | (5,275) | | | (3,099) | | | 18,211 | |
| Other current and noncurrent assets and liabilities | (1,970) | | | 9,659 | | | (16,487) | |
| Net cash from operating activities - continuing operations | 17,513 | | | (11,407) | | | 22,991 | |
| Net cash from operating activities - discontinued operations | (2,169) | | | (2,130) | | | — | |
| Net cash from operating activities | 15,344 | | | (13,537) | | | 22,991 | |
| Investing activities: | | | | | |
| Acquisitions, net of cash acquired | — | | | — | | | (262,870) | |
| Proceeds from sale of a business | 34,675 | | | — | | | — | |
| Purchase of property and equipment | (7,362) | | | (10,042) | | | (13,520) | |
| Investments and acquisition of distribution rights | — | | | (1,478) | | | (7,370) | |
| Other | — | | | (75) | | | — | |
| Net cash from investing activities - continuing operations | 27,313 | | | (11,595) | | | (283,760) | |
| Net cash from investing activities - discontinued operations | (11,506) | | | (104,841) | | | — | |
| Net cash from investing activities | 15,807 | | | (116,436) | | | (283,760) | |
| Financing activities: | | | | | |
Proceeds from issuance of Class A common stock sold in initial public offering,
net of underwriting discounts and offering costs | — | | | — | | | 107,777 | |
| Proceeds from issuance of Class A and B common stock | 778 | | | 5,822 | | | 1,633 | |
| Registration fees for Class A common stock to purchase Misonix | — | | | — | | | (1,838) | |
| Tax withholdings on equity-based compensation | — | | | (3,352) | | | — | |
| Borrowing on revolver | 64,000 | | | 25,000 | | | 20,000 | |
| Payment on revolver | (49,000) | | | (25,000) | | | (20,000) | |
| Proceeds from the issuance of long-term debt, net of issuance costs | — | | | 79,659 | | | 257,453 | |
| Debt financing costs | (3,661) | | | — | | | — | |
| Payments on long-term debt | (38,264) | | | (20,038) | | | (91,250) | |
| Other, net | (506) | | | (15) | | | (404) | |
| Net cash from financing activities | (26,653) | | | 62,076 | | | 273,371 | |
| Effect of exchange rate changes on cash | 629 | | | 521 | | | (228) | |
| Net change in cash, cash equivalents and restricted cash | 5,127 | | | (67,376) | | | 12,374 | |
| Cash, cash equivalents and restricted cash at the beginning of the period | 31,837 | | | 99,213 | | | 86,839 | |
| Cash, cash equivalents and restricted cash at the end of the period | $ | 36,964 | | | $ | 31,837 | | | $ | 99,213 | |
| Supplemental disclosure of noncash investing and financing activities | | | | | |
| Accrued liabilities for distribution rights and member distributions | $ | 709 | | | $ | — | | | $ | 3,181 | |
| Accounts payable for purchase of property, plant and equipment | $ | 1,311 | | | $ | 419 | | | $ | 695 | |
| | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
BIOVENTUS LLC
Consolidated statements of cash flows
Years ended December 31, 2020 and 2019
(Amounts in thousands)
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Operating activities: | | | | | |
| Net income (loss) | $ | 14,722 | | | $ | 6,298 | | | $ | (12,207) | |
| Net loss from discontinued operations | — | | | 1,815 | | | 16,650 | |
| Net income from continuing operations | 14,722 | | | 8,113 | | | 4,443 | |
Adjustments to reconcile net income to net cash provided by operating activities
from continuing operations: | | | | | |
| Depreciation and amortization | 28,643 | | | 30,316 | | | 29,238 | |
| Loss on impairment of intangible assets | — | | | — | | | 489 | |
| Change in fair value of contingent consideration | — | | | — | | | (739) | |
Payment of contingent consideration in excess of amount established
in purchase accounting | — | | | (945) | | | (3,558) | |
| Provision for expected credit losses | 1,215 | | | 2,242 | | | 2,538 | |
| Profits interest plan, liability-classified and other equity awards compensation | 10,103 | | | 10,844 | | | 14,325 | |
| Change in fair value of Equity Participation Rights unit | 644 | | | 565 | | | 1,009 | |
| Change in fair value of interest rate swap | 1,599 | | | — | | | — | |
| Deferred income taxes | (511) | | | (348) | | | (79) | |
| Amortization of debt discount and capitalized loan fees, net | 543 | | | 1,583 | | | 1,686 | |
| Loss on debt retirement and modification | — | | | 3,352 | | | — | |
| Other, net | (67) | | | 395 | | | 106 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable | (3,941) | | | (14,909) | | | (12,130) | |
| Inventories | (528) | | | (1,427) | | | 3,256 | |
| Accounts payable and accrued expenses | 20,510 | | | 6,646 | | | 12,148 | |
| Other current assets and liabilities | (733) | | | (3,882) | | | (422) | |
| Net cash provided by operating activities from continuing operations | 72,199 | | | 42,545 | | | 52,310 | |
| Net cash used in operating activities of discontinued operations | (400) | | | (1,832) | | | (7,123) | |
| Net cash provided by operating activities | 71,799 | | | 40,713 | | | 45,187 | |
| Investing activities: | | | | | |
| Investment and acquisition of distribution rights | (16,579) | | | (6,000) | | | (3,500) | |
| Acquisition of VIE | — | | | 430 | | | — | |
| Purchase of property and equipment | (4,093) | | | (2,342) | | | (2,561) | |
| Net cash used in investing activities from continuing operations | (20,672) | | | (7,912) | | | (6,061) | |
| Net cash provided by (used in) investing activities from discontinued operations | 172 | | | — | | | (40) | |
| Net cash used in investing activities | (20,500) | | | (7,912) | | | (6,101) | |
| Financing activities: | | | | | |
| Borrowing on revolver | 49,000 | | | — | | | — | |
| Payment on revolver | (49,000) | | | — | | | — | |
| Proceeds from the issuance of long-term debt, net of issuance costs | — | | | 198,134 | | | — | |
| Payments on long-term debt | (10,000) | | | (199,500) | | | (5,250) | |
| Other financing activities | 317 | | | (448) | | | (160) | |
| Distribution to members | (19,886) | | | (9,137) | | | (7,846) | |
| Net cash used in financing activities | (29,569) | | | (10,951) | | | (13,256) | |
| Effect of exchange rate changes on cash | 589 | | | (104) | | | (160) | |
| Net change in cash and cash equivalents | 22,319 | | | 21,746 | | | 25,670 | |
| Cash and cash equivalents at the beginning of the period | 64,520 | | | 42,774 | | | 17,104 | |
| Cash and cash equivalents at the end of the period | $ | 86,839 | | | $ | 64,520 | | | $ | 42,774 | |
| Supplemental disclosure of cash flow information | | | | | |
| Cash paid for income taxes | $ | 1,541 | | | $ | 1,577 | | | $ | 1,944 | |
| Cash paid for interest | $ | 7,486 | | | $ | 15,450 | | | $ | 17,273 | |
| Supplemental disclosure of noncash investing and financing activities | | | | | |
| Accrued liabilities for distribution rights | $ | 1,000 | | | $ | — | | | $ | 6,000 | |
| Debt conversion | $ | 973 | | | $ | — | | | $ | — | |
| Accounts payable for purchase of property and equipment | $ | 336 | | | $ | 34 | | | $ | 184 | |
| Accrued member distribution | $ | 31 | | | $ | 499 | | | $ | 906 | |
The accompanying notes are an integral part of these consolidated financial statements
BIOVENTUS LLCBioventus Inc.
Notes to consolidated financial statementsthe Consolidated Financial Statements
(Amounts in thousands, except unit and share per unit and per share data)amounts)
1. Organization and basis of presentation of financial information
The Company
Bioventus Inc. (together with its subsidiaries, the “Company”) was formed as a Delaware corporation for the purpose of facilitating an initial public offering and other related transactions in order to carry on the business of Bioventus LLC and its subsidiaries (the Company, we, us(“BV LLC”). Bioventus Inc. functions as a holding company with no direct operations, material assets or our),liabilities other than the equity interest in BV LLC. BV LLC is a limited liability company formed under the laws of the state of Delaware on November 23, 2011 and operates as a partnership. The CompanyBV LLC commenced operations in May 2012 in Durham, North Carolina, USA,2012.
On February 16, 2021, the Company completed its initial public offering (“IPO”), which was conducted through what is commonly referred to as an umbrella partnership C Corporation (“UP-C”) structure. The Company has majority interest, sole voting interest and controls the management of BV LLC. As a result, the Company consolidates the financial results of BV LLC and reports a noncontrolling interest representing the interest of BV LLC held by its headquarters. Bioventus is a global medical device company, conducting business in various countries, primarily in North America and Europe, with approximately 700 employees. continuing LLC owner.
The Company is focused on developing and commercializing clinically differentiated, cost efficient and minimally invasive treatments that engage and enhance the body’s natural healing processes.
Bioventus Inc. was formed as a Delaware corporation for the purpose of facilitating an initial public offering (IPO) The Company is headquartered in Durham, North Carolina and other related transactions in order to carry on the business of the Company. Bioventus Inc. acquired, by merger, ten entities that were members of the Company (Former LLC Owners) and upon consummation of the merger, owned 31,838,589 Common LLC Interests (as defined below). On February 16, 2021, Bioventus Inc. (New LLC Owner) closed an IPO of 9,200,000 shares of Class A common stockhas approximately 970 employees at a public offering price of $13.00 per share, which includes 1,200,000 shares issued pursuant to the underwriters' over-allotment option. The New LLC Owner received $111,228 in proceeds, net of underwriting discounts and commissions which was used to make a capital contribution to the Company in exchange for 9,200,000 newly-issued membership interests (Common LLC Interests) from the Company at a price per interest equal to the IPO price of $13.00. Subsequent to the IPO and related transactions that occurred in connection with the IPO (Transactions), the New LLC Owner is the sole managing member of the Company. Following the completion of the IPO and Transactions, the New LLC Owner owns 72.2% of the Company. The New LLC Owner has a majority economic interest, the sole voting interest in, and controls the management of the Company. As a result, subsequent to the IPO, the New LLC Owner will consolidate the financial results of the Company and will report a non-controlling interest representing the interests owned by the only member of BV LLC that remained a member (Continuing LLC Owner). Refer to Note 17. Subsequent Events for further details regarding the IPO and related transactions.December 31, 2023.
Interim periods
The Company reports quarterly interim periods on a 13-week basis within a standard calendar year. Each annual reporting period begins on January 1 and ends on December 31. Each quarter ends on the Saturday closest to calendar quarter-end, with the exception of the fourth quarter, which ends on December 31. The 13-week quarterly periods for fiscal year 2023 ended on March 28, June 27April 1, July 1 and September 2630. Comparable periods for the year2022 ended December 31, 2020. As a result, theon April 2, July 2 and October 1. The fourth and first quarters may vary in length depending on the calendar year.
PrinciplesCorrection of consolidationimmaterial misstatements
During the quarter ended April 1, 2023 and as part of the balance sheet review process, the Company identified misstatements in its calculation of the carrying amount of noncontrolling interest as it applies to the Company’s complex UP-C tax and ownership structure as prescribed in the amended and restated limited liability company agreement of BV LLC. Specifically, the Company failed to adjust the carrying amount of its noncontrolling interest to reflect changes in ownership interests relating to BV LLC. As a result, the previously issued consolidated financial statements reflect an understatement of noncontrolling interest and an overstatement of additional paid-in capital.
As a result of further research conducted, the Company discovered an additional error related to historical deferred income tax balances. The Company concluded that it had inappropriately calculated deferred income taxes by using an incorrect book basis in its investment of BV LLC during the Company’s IPO, which resulted in an overstatement of deferred tax liabilities, an understatement of noncontrolling interest and an understatement of additional paid-in capital.
The statements affected by these errors include the consolidated balance sheets and consolidated statements of stockholders’ and members’ equity issued in the Company’s Annual Report on Form 10-K for the years ended December 31, 2022 and December 31, 2021. There was no impact to any other financial statements for the periods presented. The Company concluded that these misstatements were not material, individually or in the aggregate, as evaluated under the Securities and Exchange Commission Staff Bulletin No. 99, Materiality; No. 108, Considering the Effects of Prior Year Misstatements in Current Year Financial Statements; and Financial Accounting Standards Board ASC 250-10, Accounting Changes and Error Corrections. However, because of the significance of these items, and to facilitate comparison among periods, the Company decided to revise its previously issued consolidated financial statements on a prospective basis. The Company corrected its prior period presentation for this error in its 2023 quarterly financial statements included in its Forms 10-Q and 2023 Annual Report on Form 10-K for the period ended December 31, 2023. The adjustments did not have an impact on revenues, total assets or cash flows.
The following are selected line items from our aforementioned impacted financial statements illustrating the effect of the error corrections thereon:
| | | | | | | | | | | | | | | | | |
| Consolidated Balance Sheets — December 31, 2022 | As Previously Reported | | Adjustments | | As Adjusted |
Deferred income taxes(b)(d)(e) | $ | 74,138 | | | $ | (71,890) | | | $ | 2,248 | |
| Total liabilities | 1,032,317 | | | (71,890) | | | 960,427 | |
Additional paid-in capital(a)(b)(c)(d) | 481,919 | | | 8,657 | | | 490,576 | |
Noncontrolling interest(a)(c) | 23,751 | | | 63,233 | | | 86,984 | |
| Total stockholders’ equity | 340,332 | | | 71,890 | | | 412,222 | |
| | | | | | | | | | | | | | | | | |
| Consolidated Balance Sheets — December 31, 2021 | As Previously Reported | | Adjustments | | As Adjusted |
Deferred income taxes(b) | $ | 133,518 | | | $ | (73,867) | | | $ | 59,651 | |
| Total liabilities | 692,073 | | | (73,867) | | | 618,206 | |
Additional paid-in capital(a)(b) | 465,272 | | | 8,046 | | | 473,318 | |
Noncontrolling interest(a) | 74,865 | | | 65,821 | | | 140,686 | |
| Total stockholders’ equity | 533,789 | | | 73,867 | | | 607,656 | |
The Company’s consolidated statements of changes in stockholders’ and members equity as of December 31, 2022 and December 31, 2021 have been corrected to reflect the above adjustments. The Company revised the amounts originally reported for the years ended December 31, 2022 and December 31, 2021 for the following items:
(a)Recorded a $65,821 decrease to additional paid-in capital and a corresponding increase to noncontrolling interest. This action effectively rebalanced equity appropriately between the Company and its noncontrolling interests according to their respective BV LLC ownership interests.
(b)Recorded a $73,867 decrease to deferred income tax balances and an increase to additional paid in capital to reflect the correction of an error that occurred during the calculation of deferred taxes at the Company’s IPO.
(c)Reflects the entry as discussed in (a) above and additional rebalancing activity of $2,588 relating to the issuance of Class A common stock for equity plans during the year ended December 31, 2022.
(d)Reflects the entry as discussed in (b) and an additional $1,977 increase to deferred income tax balances and a reduction to additional paid in capital to reflect the deferred tax impact during the year ended December 31, 2022.
(e)The previously reported amount reflects a reclassification of $79,863 of deferred tax liabilities associated with the deconsolidation of CartiHeal (2009) Ltd. into long-term liabilities attributable to discontinued operations as illustrated in the December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for further details.
Consolidation
The consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (U.S. GAAP)(“U.S. GAAP”). The consolidated financial statements include the Company, its subsidiaries and investments in which the Company has control. Amounts pertaining to the non-controlling ownership interests held by third parties in the operating results and financial position of the Company’s controlled subsidiaries are reported as non-controlling interests. All intercompany balances and transactions have been eliminated in consolidation.
Reclassifications
Certain prior period amounts have been reclassified to conform to the current period presentation. These changes had no effect on previously reported total revenues, net (loss) income, (loss), other comprehensive (loss) income, (loss), members’ equity or cash flows. Unless otherwise noted, all financial information in the consolidated financial statement footnotes reflectreflects the Company’s results from continuing operations. Discontinued operations are discussed further in Note 16. Discontinued operations.
Segment reporting
The Company identifies a business as an operating segment if: (i) it engages in business activities from which it may earn revenues and incur expenses; (ii) its operating results are regularly reviewed by the Chief Operating Decision Maker (CODM), to make decisions about resources to be allocated to the segment and assess its performance; and (iii) it has available discrete financial information. The Company’s CODM is its President and Chief Executive Officer. The CODM reviews financial information at the operating segment level to allocate resources and to assess the operating results and financial performance for each operating segment.
The Company’s two reportable segments are U.S. and International. U.S. and International products are primarily sold to physicians spanning the orthopedic continuum, including sports medicine, total joint reconstruction, hand and upper extremities, foot and ankle, podiatric surgery, trauma, spine and neurosurgery, as well as directly to their patients. Refer to Note 13. Revenue recognition and Note 14. Net sales and Note 15. Segments for further information regarding ourthe Company’s business segments.
Use of estimates
The preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities, at the date of the financial statements, as well as the reported amounts of revenues and expenses during the period. On an ongoing basis, management evaluates these estimates, including those related to contractual allowances and sales incentives, allowancesallowance for doubtful accounts,credit losses, inventory reserves, goodwill and intangible assets impairment, valuation of assets and liabilities assumed in acquisitions, useful lives of long lived assets, noncontrolling interest, fair value measurements, litigation and contingent liabilities, income taxes, and equity-based compensation. Management bases its estimates on historical experience, future expectations and other relevant assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from those estimates.
COVID-19 pandemic impact
In 2020, the COVID-19 pandemic spread around the world and in the U.S. and, more recently, new variants of the virus have emerged, some of which have shown to be more contagious. The COVID-19 pandemic has had widespread, rapidly evolving and unpredictable impacts on global society, economies, financial markets and business practices. Federal and state governments have implemented measures in an effort to prevent or minimize the spread of the virus, and ongoing effects of the pandemic, including social distancing, travel restrictions, border closures, limitations on public gatherings, mandatory closure or reduced capacity of business, work from home, supply chain logistical changes and other measures, which have caused global business disruptions and significant volatility in U.S. and international debt and equity markets. Our business, results of operations and financial condition have been and may continue to be, materially impacted due to the decrease in patient visits and elective procedures and any future temporary cessations of elective procedures and could be further impacted by delays in payments from customers, supply chain interruptions, extended “shelter-in-place” orders or advisories, facility closures or other reasons related to the pandemic. Furthermore, the long-term impact of COVID-19 on our business will depend on many factors, including, but not limited to, the duration and severity of the pandemic, new and ongoing measures taken in response to the pandemic, the availability and effectiveness of vaccines to combat COVID-19, the impact on economic activity from the pandemic and actions taken in response and the resulting impact it has on our partners, patients and communities in which we operate, all of which continue to be uncertain. For example, there has been a decrease in patient visits to hospitals due to risk and fear of exposure to COVID-19, as well as decreases in, or temporary moratoriums on, elective procedures, which may be re-imposed in the future. In addition to lower sales, we have experienced decreased costs as a result of the pandemic including declines in travel and lower compensation related expenses. We have also implemented other various cost reduction initiatives and measures to safeguard liquidity. As of the date of issuance of these consolidated financial statements, the extent to which COVID-19 could materially impact the Company’s financial conditions, liquidity or results of operations is uncertain.
To the extent COVID-19 disruptions continue to adversely impact our business, results of operations and financial condition, it may also have the effect of heightening risks relating to our ability to successfully commercialize new developed or acquired products or therapies, consolidation in the healthcare industry, intensified pricing pressure as a result of changes in the purchasing behavior of hospitals and maintenance of our numerous contractual relationships.
On March 27, 2020, the Coronavirus Aid, Relief and Economic Security Act (CARES Act)(“CARES Act”) was signed into law, which was aimed at providing emergency assistance and health care for individuals, families, and businesses affected by the COVID-19 pandemic and generally supporting the U.S. economy. The CARES Act, among other things, includesincluded provisions relating to refundable payroll tax credits, deferment of employer social security payments, net operating lossNOL carryback periods, alternative minimum tax credit refunds, and modifications to the net interest deduction limitations. The CARES Act allowed the Company to defer $1,889 in employer social security payroll tax payments from May 2020 through December 31, 2020. A total of 50% is2020 totaling $1,889. The Company repaid $1,440 in both December 2022 and 2021, including payments on deferred until December 31, 2021payroll tax balances acquired in business combinations.
2. Significant accounting policies
Recently issued accounting pronouncements
In addition to being a smaller reporting company and has been recorded in accrued liabilities, with the remaining balance deferred until December 31, 2022 which has been recorded in other long-term liabilities both on the consolidated balance sheet. Refer to Note 10. Income Taxes for further information.
As a result of the CARES Act and at the direction of the U.S. Department of Health and Human Services (HHS),an emerging growth company, the Company received a $1,247 Provider Relief Fund Payment in April 2020. The Company determined it complied with the conditions to be able to keepalso is an accelerated filer under SEC rules and use the funds to reimburse for health care related expenses and lost revenue attributable to the public health emergency resulting from COVID-19. A second Provider Relief Fund Payment totaling $2,854 was received in July 2020. The payments were recorded as other income on the consolidated statement of operations and comprehensive income (loss) for the year ended December 31, 2020.
2. Summary of significant accounting policies
Recent accounting pronouncements
The Company has elected to comply with non-accelerated public company filer effective dates of adoption.regulations. Therefore, the required effective dates for adopting new or revised accounting standards as described below are specific to non-accelerated public company filers, which are generally earlier than when smaller reporting companies and emerging growth companies who are not accelerated filers are required to adopt.
In December 2023, the Financial Accounting Pronouncements Recently Adopted
The FASBStandards Board (“FASB”) issued Accounting Standards Update 2016-13, Financial Instruments-Credit Losses (ASU 2016-13)2023-09 (“ASU 2023-09”), in June 2016 that significantly changes accountingIncome Taxes, which enhances the transparency of income tax disclosures by expanding annual disclosure requirements related to the rate reconciliation and income taxes paid. The amendments are effective for credit losses for most financial assets and certain other instruments that are not measured at fair value through net income. ASU 2016-13 requires that an entity measure and recognize expected credit losses for financial assets held at amortized cost and replaces the incurred loss impairment methodology in prior GAAP withfiscal years beginning after December 15, 2024. Early adoption is permitted. The amendments should be applied on a methodology that considers a broad range of information for the estimation of credit losses.prospective basis. Retrospective application is permitted. The Company adoptedis currently evaluating this ASU 2016-13 on January 1, 2020 prospectively and the adoption did not have a materialto determine its impact on the Company’s consolidated financial statements.Company's disclosures.
In August 2018,November 2023, the FASB issued Accounting Standards Update 2018-15, Intangibles-Goodwill2023-07 (“ASU 2023-07”), Segment Reporting, which improves reportable segment disclosure requirements. ASU 2023-07 primarily enhances disclosures about significant segment expenses by requiring that a public entity disclose significant segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and Other-Internal-Use Software (ASU 2018-15), addressingincluded within each reported measure of segment profit or loss. This ASU also (i) requires that a customer’s accountingpublic entity disclose, on an annual and interim basis, an amount for implementation costs incurredother segment items by reportable segment, and a description of its composition; (ii) requires that all annual disclosures are provided in a cloud-computing arrangement (CCA)the interim periods; (iii) clarifies that is considered a service contract. Under ASU 2018-15, implementation costs for a CCA shouldif the CODM uses more than one measure of profitability in assessing segment performance and deciding how to allocate resources, that one or more of those measures may be evaluated for capitalization using the same approach as implementation costs associated with internal-use software. The capitalized implementation costs should be expensed over the termreported; (iv) requires disclosure of the hosting arrangement, which includes any reasonably certain renewal periods. Capitalized implementation costs should be assessedtitle and position of the CODM and a description of how the reported measures are used by the CODM in assessing segment performance and in deciding how to allocate resources; (v) requires that an entity with a single segment provide all new required disclosures. ASU 2023-07 is effective for impairment like long-lived assets.fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024 and requires retrospective application. Early adoption is permitted. The Company adoptedamendments under ASU 2018-15 on January 1, 2020 prospectively2023-07 relate to financial disclosures and it had no materialits adoption will not have an impact on the Company’s consolidatedresults of operations, financial statements.
In August 2018, the FASB issued Accounting Standards Update 2018-13, Fair Value Measurement (ASU 2018-13), modifying the disclosure requirements on fair value measurements and eliminates the requirement to disclose the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, the policy for timing of transfers between levels and the valuation processes for Level 3 fair value measurements. ASU 2018-13 modifies certain disclosures related to investments measured at net asset value and clarifies that companies are to disclose uncertainties in measurements as of the reporting date. ASU 2018-13 requires additional disclosure related to changes in unrealized gains and losses included in other comprehensive income (loss) for recurring Level 3 fair value measurements as well as the range and weighted average,position or other quantitative information that would be a more reasonable and rational method, of significant unobservable inputs used to develop Level 3 fair value measurements.cash flows. The Company adoptedwill adopt ASU 2018-13 on January 1, 2020 and it did not have a material impact on the Company’s consolidated financial statements.
New Accounting Pronouncements Not Yet Adopted
In December 2019, the FASB issued Accounting Standards Update 2019-12, Income Taxes (ASU 2019-12), which amended the accounting2023-07 for income taxes. ASU 2019-12 eliminates certain exceptions to the guidance for income taxes related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences as well as simplifying aspects of the accounting for franchise taxes and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. ASU 2019-12 is effective for annual and interim periods beginning after December 15, 2020. Early adoption is permitted in interim or annual periods for which financial statements have not been made available for issuance. Entities that elect to early adopt the amendments in an interim period should reflect any adjustments as of the beginning of the annual reporting period that includes thatending December 31, 2024 and for interim period. Certain amendments are to be applied prospectively while others are retrospective. The Company adopted ASU 2019-12 on January 1, 2021 and the Company has assessed it will not have a material impact on its consolidated financial statements.reporting periods thereafter.
Variable Interest Entity
The Company reviews each investment and collaboration agreement to determine if it has a variable interest in the entity. In assessing whether the Company has a variable interest in the entity as a whole, the Company considers and makes judgments regarding the purpose and design of the entity, the value of the licensed assets to the entity, the value of the entity’s total assets and the significant activities of the entity. If the Company has a variable interest in the entity as a whole, the Company assesses whether or not the Company is a primary beneficiary of that variable interest entity (VIE)(“VIE”), based on a number of factors, including: (i) which party has the power to direct the activities that most significantly affect the VIE’s economic performance, (ii) the parties’ contractual rights and responsibilities pursuant to the collaboration agreement, and (iii) which party has the obligation to absorb losses of or the right to receive benefits from the VIE that could be significant to the VIE. If the Company determines that it is the primary beneficiary of a VIE at the onset of the collaboration, the collaboration is treated as a business combination and the Company consolidates the financial statement of the VIE into the Company’s consolidated financial statements. On a quarterly basis, the Company evaluates whether it continues to be the primary beneficiary of the consolidated VIE. If the Company determines that it is no longer the primary beneficiary of a consolidated VIE, it deconsolidates the VIE in the period the determination is made.
Assets and liabilities recorded as a result of consolidating financial results of the VIE into the Company’s consolidated balance sheet do not represent additional assets that could be used to satisfy claims against the Company’s general assets or liabilities for which creditors have recourse to the Company’s general assets.
Noncontrolling Interest
The Company records noncontrolling interest related to the consolidated VIEs on its consolidated balance sheet.sheet related to the economic interest in BV LLC held by the only continuing BV LLC owner as well as consolidated VIEs. The Company records loss attributable to noncontrolling interest on its consolidated statements of operations, which reflects the VIE’s net loss for the reporting period, adjusted for changes in the noncontrolling interest holders claim to net assets, including contingent milestone and royalty payments, which are evaluated each reporting period.
Deconsolidation and discontinued operations
Upon the occurrence of certain events and on a regular basis, the Company evaluates whether it no longer has a controlling interest in its subsidiaries, including consolidated VIEs. If the Company determines it no longer has a controlling interest, the subsidiary is deconsolidated. The Company records a gain or loss on deconsolidation based on the difference on the deconsolidation date between (i) the aggregate of (a) the fair value of any consideration received, (b) the fair value of any retained noncontrolling investment in the former subsidiary and (c) the carrying amount of any noncontrolling interest in the subsidiary being deconsolidated, less (ii) the carrying amount of the former subsidiary’s assets and liabilities.
The Company assesses whether a deconsolidation is required to be presented as discontinued operations in its consolidated financial statements on the deconsolidation date. This assessment is based on if the deconsolidation represents a strategic shift that has or will have a major effect on the Company’s operations or financial results. If the Company determines that a deconsolidation requires presentation as a discontinued operation on the deconsolidation date, or at any point during the one-year period following such date, it will present the former subsidiary as a discontinued operation for all periods presented.
Effect of foreign currency
The assets and liabilities of foreign subsidiaries whose functional currency is the local currency are translated into U.S. dollars at rates of exchange in effect at the close of their month end. Equity accounts are translated at their historical rates. Revenues and expenses are translated at the exchange rate on the transaction date. Translation gains and losses are accumulated within accumulated other comprehensive (loss) income (loss) as a separate component of members’ equity.
Foreign currency transaction gains and losses are included in other expense on the consolidated statements of operations and other comprehensive income (loss). income. There were gainswas a gain of $117$351 for the year ended December 31, 2020, nominal2023 and losses of $250 and $132 for the yearyears ended December 31, 2019,2022 and losses of $234 for the year ended December 31, 2018
Other comprehensive income (loss)2021, respectively.
Comprehensive (loss) income
Comprehensive (loss) income consists of two components: net (loss) income (loss) and other comprehensive (loss) income, (loss). Other comprehensive income (loss)which refers to gains and losses that are recorded under U.S. GAAP are recorded as an element of members’stockholders’ equity and are excluded from net income (loss). income. The Company’s other comprehensive (loss) income (loss) consists of a defined benefit plan adjustment and foreign currency translation adjustments from those subsidiaries not using the U.S. dollar as their functional currency.
Cash, and cash equivalents and restricted cash
Cash equivalents consist of highly liquid investments with an original maturity of three months or less at date of purchase. The Company’s cash is primarily held in financial institutions in the United States and the Netherlands. The Company maintains cash balances in the United States in excess of the federally insured limits. TheRestricted cash is cash the Company didholds for specific reasons and is not have restricted cash as of December 31, 2020 and 2019.available for immediate business use.
Derivatives
The Company useshas historically used derivative instruments to manage exposures to interest rates. Derivatives are recorded on the balance sheet at fair value at each balance sheet date and the Company does not designate whether the derivative instrument is an effective hedge. Changes in the fair values of derivative instruments are recognized in the consolidated statements of operations and comprehensive income (loss). income. The Company hashad entered, and may in the future enter, into derivative contracts related to its debt. Refer to Note 5. Financial instruments for further details regarding the Company’s derivatives.
Fair value
The Company records certain assets and liabilities at fair value. Refer to Note 7.6. Fair value measurements for details regarding assets and liabilities measured at fair value. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy that prioritizes the inputs used to measure fair value is described below. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. Assets and liabilities are categorized based on the lowest level that is significant to the valuation.
The three levels of inputs used to measure fair value are as follows:
•Level 1—Quoted prices in active markets for identical assets or liabilities;
•Level 2—Observable inputs other than quoted prices included within Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data; and
•Level 3—Unobservable inputs that are supported by little or no market data. This includes certain pricing models, discounted cash flow methodologies and similar techniques that use significant unobservable inputs.
Revenue recognition
Sale of Products
The Company derives revenue primarily from the sale ofproduct sales in its (i) Pain Treatments portfolio, which includes osteoarthritic or OA, joint(OA) pain treatment and joint preservation products,treatments, which are hyaluronic acid or HA,(“HA”), viscosupplementation therapies and peripheral nerve stimulation products, (ii) Bone Graft Substitutes (BGS)Surgical Solutions portfolio, which includes bone graft substitutes, tissue resection, ultrasonic bone cutting and sculpting systems and other surgical products, and (iii) a Minimally Invasive Fracture Treatment product.Restorative Therapies portfolio, which includes minimally invasive fracture treatments and rehabilitation products. The Company sells product directly to healthcare institutions, patients, distributors and dealers. The Company also enters arrangements with pharmacy and health benefit managers that provide for privately negotiated rebates, chargebacks and discounts with respect to the purchase of the Company’s products.
The Company recognizes revenue generally at a point in time upon transfer of control of the promised product to customers in an amount that reflects the consideration it expects to receive in exchange for those products. The Company excludes taxes collected from customers and remitted to governmental authorities from revenues.
Revenues are recorded at the transaction price, which is determined as the contracted price net of estimates of variable consideration resulting from discounts, rebates, returns, chargebacks, contractual allowances, estimated third-party payer settlements, and certain distribution and administration fees offered in our customer contracts and other indirect customer contracts relating to the sale of our products. The Company establishes reserves for the estimated variable consideration based on the amounts earned or eligible to be claimedfor claim on the related sales. Where appropriate, these estimates take into consideration a range of possible outcomes, which are probability-weighted for relevant factors such as the Company’s historical experience, current contractual requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. The amount of variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period. The Company regularly reviews all reserves and updates them at the end of each reporting period as needed. There were no significant adjustments arising from the change in estimates of variable consideration that were significant for the years ended December 31, 2020, 20192023, 2022 and 2018.2021.
OA Joint Pain Treatment and Joint PreservationTreatments
Revenue from customers, such as healthcare providers, distribution centers or specialty pharmacies is recognized at the point in time when control is transferred to the customer, typically upon shipment.
Distributor chargebacks
The Company has preexisting contracts with established rates with many of the distributors’ customers whothat require the distributors to sell our productproducts at their established rate. The Company offers chargebacks to distributors who supply these customers with our products. The Company reduces revenue at the time of sale for the estimated future chargebacks. The Company records chargeback reserves as a reduction of accounts receivable and basebases the reserves on the expected value by using probability-weighted estimates of volume of purchases, inventory holdings and historical chargebacks requested for each distributor.
Discounts and gross-to-net deductions
The Company offers retrospective discounts and gross-to-net deductions linked to the volume of purchases which may increase at negotiated thresholds within a contract-buying period. The Company reduces revenue and records the reserve as a reduction to accounts receivable for the estimated discount and rebate at the expected amount the customer will earn, based on historical buying trends and forecasted purchases.
Bone Graft SubstituteSurgical Solutions
Most of the Company’s BGS product sales related to bone graft substitutes are through consignment inventory with hospitals, where ownership remains with the Company until the hospital or ambulatory surgical center (ASC)(“ASC”) performs a surgery and consumes the consigned inventory. The Company recognizes revenue when the surgery has been performed. Control of the product is not transferred until the customer consumes it, as the Company is able to require the return or transfer of the product to a third-party.third-party prior to the products use. An unconditional obligation to pay for the product does not exist until the customer consumesuses it.
Minimally Invasive Fracture TreatmentThe Company consistently recognizes revenue from sales of its ultrasonic products in accordance with shipping terms. Control is transferred to the customer when the product is shipped or received, and revenue is recognized accordingly.
Restorative Therapies
The Company recognizes revenue from third-party payers, such as governmental agencies, insurance companies or managed care providers, when the Company transfers control to the patient, typically when the patient has accepted the product or upon delivery. The Company records this revenue at the contracted rate, net of contractual allowances and estimated third-party payer settlements at the time of sale, or an estimated price based on historical data and other available information for non-contracted payers. The Company estimates the contractual allowances using the portfolio approach and based on probability weighting historical data and collections history within those portfolios. The portfolios determined using the portfolio approach consist of the following customer groups: government payers, commercial payers, and patients. The Company recognizes revenue from patients (self-pay and insured patients with coinsurance and deductible responsibilities) based on billed amounts giving effect to any discounts and implicit price concessions. Implicit price concessions represent differences between amounts billed and the amounts the Company expects to collect from patients, which considers historical collection experience and current market conditions.
Settlements with third-party payers for retroactive revenue adjustments due to audits, reviews or investigations are considered variable consideration and are included in the determination of the estimated transaction price using the expected amount method. These settlements are estimated based on the terms of the payment agreement with the payer, correspondence from the payer and historical settlement activity, including an assessment to ensure that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the retroactive adjustment is subsequently resolved. Estimated settlements are adjusted in future periods as adjustments become known (that is, new information becomes available), or as years are settled or are no longer subject to such audits, reviews and investigations. The Company is not aware of any claims, disputes or unsettled matters with any payer that would materially affect revenues for which the Company has not adequately provided for or disclosed in the accompanying consolidated financial statements. Refer to Note 12. Commitments and contingencies for further information.
The Company recognizes revenue from patients (self-pay and insured patients with coinsurance and deductible responsibilities) based on billed amounts giving effect to any discounts and implicit price concessions. Implicit price concessions represent differences between amounts billed and the amounts the Company expects to collect from patients, which considers historical collection experience and current market conditions. The Company recognizes revenue from other restorative therapies products generally at the point in time when control is transferred to the customer, either upon shipment or reaching the destination, depending on the product.
Product returns
The Company estimates the amount of returns and reduces revenue in the period the related product revenue is recognized. The Company records a liability for expected returns based on probability-weighted historical data.
Accounts receivable, net
Accounts receivable, net are amounts billed and currently due from customers. The Company records the amounts due net of allowance for doubtful accounts.credit losses. The Company maintains an estimated allowance for doubtful accountscredit losses to provide for receivables the Company does not expect to collect. The Company bases the allowance on an assessment of customer creditworthiness, historical payment experience, the age of outstanding receivables and other information as applicable. Collection of the consideration that the Company expects to receive typically occurs within 30 to 90 days of billing. The Company applies the practical expedient for contracts with payment terms of one year or less which does not consider the effects of the time value of money. Occasionally, the Company enters into payment agreements with patients that allow payment terms beyond one year. In those cases, the financing component is not deemed significant to the contract.
Contract assets
Contract assets consist of unbilled amounts resulting from estimated future royalties from an international distributor that exceeds the amount billed. Contract assets totaling $81$257 and $261$267 as of December 31, 20202023 and 2019,2022, respectively, are included in prepaid and other current assets on the consolidated balance sheets, respectively.sheets.
Contract liabilities
Contract liabilities consist of customer advance payments or deposits and deferred revenue. Occasionally for certain international customers, the Company requires payments in advance of shipping product and recognizing revenue resulting in contract liabilities. Contract liabilities were nominal$2,347 and $2,895 as of December 31, 20202023 and 20192022, respectively, and are included in accrued liabilities on the consolidated balance sheets.
Shipping and handling
The Company classifies amounts billed for shipping and handling as a component of net sales. The related shipping and handling fees and costs as well as other distribution costs are included in cost of sales. The Company has elected to recognize shipping and handling activities that occur after control of the related product transfers to the customer as fulfillment costs and these are included in cost of sales.
Contract costs
The Company applies the practical expedient of recognizing the incremental costs of obtaining contracts as an expense when incurred as the amortization period of the assets that the Company otherwise would have recognized is one year or less. These incremental costs include the Company’s sales incentive programs for the internal sales force and third-party sales agents as the compensation is commensurate with annual sales activities. These costs are included in selling, general and administrative expense on the consolidated statements of operations and comprehensive income (loss). income.
Inventory
The Company values its inventory at the lower of cost or net realizable value and adjusts for the value of inventory that is estimated to be excess, obsolete or otherwise unmarketable. Cost is determined using the first-in, first-out (FIFO) method. Elements of cost in inventory include raw materials, direct labor, manufacturing overhead and inbound freight. The Company records allowances for excess and obsolete inventory based on historical and estimated future demand and market conditions. Inventory items used for demonstration purposes, rentals and consigned generators are classified as property and equipment.
Business combinations
Accounting for acquisitions requires the Company to recognize separately from goodwill assets acquired and the liabilities assumed at their acquisition date fair values. Goodwill as of the acquisition date is measured as the excess of consideration transferred over the net of the acquisition date fair values of the assets acquired and the liabilities assumed. While best estimates and assumptions are used to accurately value assets acquired and liabilities assumed at the acquisition date, as well as contingent consideration where applicable, estimates are inherently uncertain and subject to refinement. During the measurement period, which may be up to one year from the acquisition date, the Company may record adjustments to the assets acquired and liabilities assumed with a corresponding offset to goodwill. Upon the conclusion of the measurement period or final determination of the values of assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded to ourwithin the consolidated statements of operations and comprehensive income (loss). income. Subsequent changes in the estimated fair value of contingent consideration are recognized in earnings in the period of change.
Long-lived assets
Property and equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization expense are recognized using the straight-line method over the estimated useful life of each asset, or the shorter of the lease term or useful life if related to leasehold improvements. The useful lives in years are as follows:
| | | | | |
Computer software and hardware | 3-5 |
Leasehold improvements | 7 |
Machinery and equipment | 7 |
Furniture and fixtures | 7 |
Goodwill and intangible assets
Finite-lived intangible assets were initially recorded at fair value upon acquisition and are amortized using the straight-line method over their estimated useful lives in years are as follows:
| | | | | |
| Weighted
Average
Useful Life
|
Intellectual property | 17.3 |
Distribution rights | 12.7 |
Customer relationships | 10.0 |
Developed technology | 8.3 |
Goodwill is not amortized but is evaluated for impairment annually or more frequently if events or changes in circumstances indicate that goodwill might be impaired. The Company reviews goodwill for impairment by applying a quantitative impairment analysis where the fair value of the reporting unit is compared with the carrying value, including goodwill. The Company determines the fair value of each reporting unit based on an income approach. The value of each reporting unit is determined on a stand-alone basis from the perspective of a market participant and represents the price estimated to be received in a sale of the reporting unit in an orderly transaction between market participants at the measurement date. The Company performs its annual goodwill impairment test on October 31st. If the fair value of the reporting unit is less than its carrying value, the Company will recognize the difference as an impairment loss, which is limited to the amount of goodwill allocated to the reporting units. There were no goodwill impairment charges for the years ended December 31, 2020, 2019 and 2018.
Software development costs
The Company capitalizes internal and external costs incurred to develop internal-use software during the application development stage for software design, configuration, coding and testing upon placing the asset in service and then amortizes these costs on a straight-line basis over the estimated useful life of the product, not to exceed three years. The Company does not capitalize costs that are precluded from capitalization in authoritative guidance, such as preliminary project phase costs, training costs or data conversion costs. Capitalized software costs totaled $17,653 and $14,119 as of December 31, 2020 and 2019 and the related accumulated amortization totaled $13,264 and $12,184, respectively. Amortization expense was $1,184, $1,138 and $1,204 for the years ended December 31, 2020, 2019 and 2018, respectively.
The carrying values of property, equipment, intangible assets as well as other long-lived and indefinite lived assets are reviewed for recoverability if the facts and circumstances suggest that a potential impairment may have occurred. If this review indicates that carrying values may not be recoverable, the Company will perform an assessment to determine if an impairment charge is required to reduce carrying values to estimated fair value. If quoted market prices are not available, the Company estimates fair value using an undiscounted value of estimated future cash flows. During 2018, the Company determined that it would no longer sell a specific BGS product and as a result, an intangible asset related to this product was fully written off and the Company recognized impairment charges of $489 for the year ended December 31, 2018, which is included in the consolidated statements of operations and comprehensive income (loss). Upon retirement or sale of property and equipment, the cost of assets disposed of and the related accumulated depreciation and amortization are removed from the accounts, and any resulting gain or loss is included in income from operations.
ThereThe Company recorded an impairment loss of $78,615 for the year ended December 31, 2023 in the U.S. reporting segment of net intellectual property attributable to the TheraSkin and TheraGenesis products, which were sold in May 2023. The loss was recorded in impairment of assets within the consolidated statements of operations and comprehensive (loss) income. During the year ended December 31, 2021, the Company recognized an impairment of $5,674 on long lived assets related to a VIE of which $5,176 is attributable to the non-controlling interests, refer to Note 4. Acquisitions and divestitures for further information regarding both of these impairments.
Except for the previously described impairments of intangibles and the Company’s VIE, there were no other events, facts or circumstances for the years ended December 31, 2020, 20192023, 2022 and 20182021 that resulted in any impairment charges to the Company’s property, equipment, intangible or other long-lived assets.
Property and equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization expense are recognized using the straight-line method over the estimated useful life of each asset, or the shorter of the lease term or useful life if related to leasehold improvements. Refer to Note 12. Commitments and contingencies for further details regarding leased assets. Depreciation of generators used with certain surgical solutions are consigned to customers and depreciation is charged to selling expenses. The useful lives in years are as follows:
| | | | | |
| Computer software and hardware | 3 - 5 |
| Demonstration and consignment inventory | 5 |
| Furniture and fixtures | 7 |
| Leasehold improvements | 7 |
| Machinery and equipment | 7 |
Intangible assets
Finite-lived intangible assets were initially recorded at fair value upon acquisition and are amortized using the straight-line method over their estimated useful lives in years are as follows:
| | | | | |
| Weighted Average Useful Life |
| Intellectual property | 18.6 |
| Distribution rights | 11.2 |
| |
| |
| Developed technology | 9.8 |
Goodwill
Goodwill is evaluated for impairment annually or more frequently if events or changes in circumstances indicate that goodwill might be impaired. The Company assesses goodwill impairment by applying a quantitative impairment analysis comparing the carrying value of the Company’s reporting units to their respective fair values. A goodwill impairment exists if the carrying value of the reporting unit exceeds its fair value.
The Company has two reporting units and assesses impairment based upon qualitative factors and if necessary, quantitative factors. A reporting unit's fair value is determined using the income approach and discounted cash flow models by utilizing Level 3 inputs and assumptions such as future cash flows, discount rates, long-term growth rates, market value and income tax considerations. Specifically, the value of each reporting unit is determined on a stand-alone basis from the perspective of a market participant and represents the price estimated to be received in a sale of the reporting unit in an orderly transaction between market participants at the measurement date. The Company performs its annual goodwill impairment test on October 31. If the fair value of the reporting unit is less than its carrying value, the Company will recognize the difference as an impairment loss, which is limited to the amount of goodwill allocated to the reporting units. There were no goodwill impairment charges for the years ended December 31, 2023 and 2021.
On November 8, 2022, due to a significant decline in the Company’s Class A common stock, circumstances became evident that a possible goodwill impairment existed as of the third quarter balance sheet date. The Company concluded that the carrying value of the U.S. reporting unit exceeded its fair value. The Company recorded a non-cash goodwill impairment charge within the U.S. reporting unit for the year ended December 31, 2022. The impairment was recorded within impairment of assets on the consolidated statements of operations and comprehensive (loss) income. Refer to Note 3. Balance sheet information for further details.
Software development costs
The Company capitalizes internal and external costs incurred to develop internal-use software during the application development stage for software design, configuration, coding and testing upon placing the asset in service and then amortizes these costs on a straight-line basis over the estimated useful life of the product, not to exceed three years. The Company does not capitalize costs that are precluded from capitalization in authoritative guidance, such as preliminary project phase costs, training costs or data conversion costs. Capitalized software costs totaled $36,782 and $31,041 as of December 31, 2023 and 2022 and the related accumulated amortization totaled $28,040 and $20,997, respectively. Amortization expense was $6,694, $4,449 and $2,227 for the years ended December 31, 2023, 2022 and 2021, respectively.
Acquired in-process research and development
The fair value of in-process research and development (IPR&D)(“IPR&D”) assets acquired in a business combination are capitalized and accounted for as indefinite-lived intangible assets and are not amortized until development is completed and the product is available for sale. Once the product is available for sale, the asset is transferred to developed technology and amortized over its estimated useful life. Impairment tests for IPR&D assets occur at least annually in December, or more frequently if events or changes in circumstances indicate that the asset might be impaired. If the fair value of the intangible assets is less than the carrying amount, an impairment loss is recognized for the difference. There were no events, facts or circumstances for the years ended December 31, 2020, 2019 and 2018 that resulted in any impairment charges to the Company’s IPR&D.
Deferred Offering Costs
Deferred offering costs, consisting of legal, accounting, filing and other fees related to the initial public offering, are capitalized. The deferred offering costs will be offset against proceeds from the initial public offering upon the effectiveness of the initial public offering. In the event the initial public offering is terminated, all capitalized deferred offering costs would be expensed. Deferred offering costs capitalized totaled $2,187 as of December 31, 2020 and there were there were no deferred offering costs capitalized as of December 31, 2019.
Concentration of risk
The Company provides credit, in the normal course of business, to its customers. The Company does not require collateral or other securities to support customer receivables. Credit losses are provided for through allowances and have historically been materially within management’s estimates.
Certain suppliers provide the Company with product that results in a significant percentage of total sales for the years ended December 31 as follows:
| | 2020 | | 2019 | | 2018 |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Supplier A | Supplier A | 26 | % | | 20 | % | | 12 | % | Supplier A | 27 | % | | 25 | % | | 27 | % |
| Supplier B | Supplier B | 17 | % | | 19 | % | | 17 | % | Supplier B | 17 | % | | 15 | % | | 15 | % |
| Supplier C | Supplier C | 10 | % | | 15 | % | | 20 | % | Supplier C | 8 | % | | 10 | % | | 15 | % |
| Supplier D | | Supplier D | 7 | % | | 7 | % | | 8 | % |
Accounts payable to these significant suppliers at December 31 were as follows:
| | 2020 | | 2019 | |
| 2023 | | | 2023 | | 2022 |
| Supplier A | Supplier A | $ | 2,983 | | | $ | 3,586 | | |
| Supplier B | Supplier B | $ | 471 | | | $ | 697 | | |
| Supplier C | Supplier C | $ | 1,000 | | | $ | 360 | | |
| Supplier D | |
Certain products provide the Company with a significant percentage of total sales for the years ended December 31 as follows:
| | 2020 | | 2019 | | 2018 |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Product A | Product A | 27 | % | | 30 | % | | 38 | % | Product A | 27 | % | | 25 | % | | 27 | % |
| Product B | Product B | 26 | % | | 20 | % | | 12 | % | Product B | 14 | % | | 14 | % | | 20 | % |
| Product C | Product C | 17 | % | | 19 | % | | 17 | % | Product C | 17 | % | | 15 | % | | 15 | % |
| Product D | Product D | 10 | % | | 15 | % | | 20 | % | Product D | 8 | % | | 10 | % | | 15 | % |
| Product E | | Product E | 7 | % | | 7 | % | | 8 | % |
Restructuring costs
The Company has restructured portions of its operations and future restructuring activities are possible. Identifying and calculating the cost to exit these operations requires certain assumptions to be made, the most significant of which are anticipated future liabilities. Although estimates have been reasonably accurate in the past, significant judgment is required, and these estimates and assumptions may change as additional information becomes available and facts or circumstances change. Restructuring costs are recorded at estimated fair value. Key assumptions in determining the restructuring costs include negotiated terms and payments to terminate contractual obligations.
Profits interestEquity-based compensation
The Company measures profits interest compensation cost for all share-based payments at the grant date based on the fair value of the award and recognizes this cost as compensation expense over the required or estimated service period forvesting period. The Company uses the Black-Scholes method to value options and the market price on the date of grant to value restricted stock. The Company utilizes the straight-line amortization method to recognize the expense associated with the awards expected to vest. Certain awards are liability-classified, which require they be remeasured at each reporting date.with graded vesting terms. Compensation expense is included in Selling,selling, general and administrative expense and Researchresearch and development expense on the consolidated statement of operations and comprehensive (loss) income (loss) based upon the classification of the employees who were granted the awards.
Advertising costs
Advertising costs include costs incurred to promote the Company’s business and are expensed as incurred.incurred and recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive (loss) income. Advertising costs were $2,769, $2,351$3,853, $5,203 and $2,916$3,873 for the years ended December 31, 2020, 20192023, 2022 and 2018,2021, respectively.
Research and development expense
Research and development expense consist primarily of employee compensation and related expenses as well as contract research organization services. Internal research and development costs are expensed as incurred. Research and development costs incurred by third parties are expensed as the contracted work is performed.
Collaborative agreements
The Company periodically enters into strategic alliance agreements with counterparties to produce products and/or provide services to customers. Alliances created by such agreements are not legal entities, have no employees, no assets and have no true operations. These arrangements create contractual rights and we accountthe Company accounts for these alliances as a collaborative arrangement by reporting costs incurred from transactions within research and development expense in ourwithin the consolidated statements of operations.
Contingencies
The Company records a liability in the consolidated financial statements on an undiscounted basis for loss contingencies when a loss is known or considered probable and the amount may be reasonably estimated. If the reasonable estimate of known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. Legal fees expected to be incurred in connection with a loss contingency are not included in the estimated loss contingency. The Company accrues for any legal costs as they are incurred.
Income taxes
The Company is treated as a partnershipaccounts for U.S. tax purposes. Accordingly,income taxes using the profitsasset and losses are passed through to the members and included in their income tax returns. The Company has been required to make tax distributions to its members in an amount equal to 40% of the members’ taxable income attributable to their ownership. The tax rate applied for purposes of this distribution may be changed only by approval of the Company’s Board of Managers.
Certain wholly owned subsidiaries of the Company are taxable entities for U.S. or foreign tax purposes and file tax returns in their local jurisdictions. Income tax expense includes U.S. federal, state and international income taxes. Certain items of income and expense are not reported in income tax returns and financial statements in the same year. The income tax effects of these differences are reported as deferred income taxes. Valuation allowances are provided to reduce the relatedliability method, under which deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial reporting and income tax basis of assets and liabilities, and for operating losses and credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates in effect for the years in which those items are expected to an amount which will,be realized. Tax law and rate changes are recorded in the period such changes are enacted. The Company establishes a valuation allowance when it is more likely than not that certain deferred tax assets will not be realized.realized in the foreseeable future.
The Company recognizes a tax benefit from any uncertain tax positions only if they are more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured as the largest amount of benefit determined on a cumulative probability basis that the Company believes is more likely than not to be realized upon ultimate settlement of the position. Components of the reserve, if relevant, are classified as a current or noncurrent liability in the consolidated balance sheet based on when the Company expects each of the items to be settled. Interest and penalties related to unrecognized tax benefits are recognized as a component of income tax expense.
Earnings per common unitshare
Basic income (loss)earnings per common unitshare is determined by dividing thecalculated using net income (loss) allocableor loss attributable to Bioventus, Inc. Class A common unit holdersstock-holders, divided by the weighted-average Class A common stock outstanding. Diluted earnings per share is calculated using net income attributable to Bioventus, Inc. Class A common stockholders, divided by the weighted average numberClass A common stock outstanding adjusted for the effect of common units outstanding duringgranted stock awards determined to be dilutive under the periods presented. Diluted loss per common unit is computed by dividing the net income (loss) allocable to common unit holders on an “if converted” basis by the weighted average number of actual common units outstanding and, when dilutive, the unit equivalents that would arise from the assumed conversion of convertible instruments, if any.treasury stock method.
Subsequent Events
The Company has considered the effects of subsequent events through March 26, 2021, the date the Company’s consolidated financial statements were issued.
3. Balance sheet information
Cash, cash equivalents and restricted cash
Cash, cash equivalents and restricted cash totaled $36,964 and $30,186 as of December 31, 2023 and 2022. Cash and restricted cash of $1,628 and $23, respectively, attributable to CartiHeal has been reclassified to current assets attributable to discontinued operations within the December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for further details regarding the deconsolidation of CartiHeal.
Accounts receivable, net
Accounts receivable, net are amounts billed and currently due from customers. The Company records the amounts due net of allowance for credit losses. Collection of the consideration that the Company expects to receive typically occurs within 30 to 90 days of billing. The Company applies the practical expedient for contracts with payment terms of one year or less which does not consider the effects of the time value of money. Occasionally, the Company enters into payment agreements with patients that allow payment terms beyond one year. In those cases, the financing component is not deemed significant to the contract.
Accounts receivable, net of allowances, consisted of the following as of December 31:
| | 2020 | | 2019 |
| Accounts receivable | $ | 92,273 | | | $ | 89,274 | |
| 2023 | | | 2023 | | 2022 |
Accounts receivable(a) | |
| Less: Allowance for credit losses | Less: Allowance for credit losses | (3,990) | | | (4,146) | |
| $ | 88,283 | | | $ | 85,128 | |
| $ | |
The Company maintains an allowance(a)Other receivables of $350 attributable to CartiHeal was reclassified to current assets attributable to discontinued operations within the December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for credit losses for estimated losses resulting fromfurther details regarding the inabilitydeconsolidation of its customers to make required payments. The allowance for credit losses is calculated by region and by customer type, where appropriate considering several factors including age of accounts, collection history, historical account write-offs, current economic conditions, and supportable forecasted economic expectations. CartiHeal.
Due to the short-term nature of its receivables, the estimate of expected credit losses is based on aging of the account receivable balances. The allowance is adjusted on a specific identification basis for certain accounts as well as pooling of accounts with similar characteristics. An increase in the provision for credit losses may be required when the financial condition of the Company’s customers or its collection experience deteriorates. The Company has a diverse customer base with no single customer representing ten percent or more of sales orsales. The Company had one customer representing approximately 16.0% and 12.1% of the accounts receivable.receivable balance as of December 31, 2023 and 2022, respectively. Historically, the Company’s reserves have been adequate to cover credit losses. The Company’s exposure to credit losses may increase if its customers are adversely affected by changes in health care laws, coverage and reimbursement, economic pressures or uncertainty associated with local or global economic recessions, disruption associated with the COVID-19 pandemic, or other customer-specific factors. The Company considered the current and expected future economic and market conditions surrounding the COVID-19 pandemic and determined that the estimate of credit losses was not significantly impacted. Estimates are used to determine the allowance, which are based on an assessment of anticipated payment and all other historical, current and future information that is reasonably available.
Changes in credit losses were as follows for the years ended December 31:
| | 2020 | | 2019 |
| | | | | 2023 | |
| | | | | 2023 | |
| | | | | 2023 | |
| Beginning balance | Beginning balance | $ | (4,146) | | | $ | (4,497) | |
| Provision for credit losses | (1,215) | | | (2,242) | |
| Beginning balance | |
| Beginning balance | |
| Benefit (provision) for expected credit losses | |
| Benefit (provision) for expected credit losses | |
| Benefit (provision) for expected credit losses | |
| Write-offs | |
| Write-offs | |
| Write-offs | Write-offs | 1,787 | | | 2,949 | |
| Recoveries | Recoveries | (416) | | | (356) | |
| Recoveries | |
| Recoveries | |
| Disposals | |
| Disposals | |
| Disposals | |
| Ending balance | Ending balance | $ | (3,990) | | | $ | (4,146) | |
| Ending balance | |
| Ending balance | |
Inventory
Inventory consisted of the following as of December 31:
| | | | | | | | | | | |
| 2020 | | 2019 |
| Raw materials and supplies | $ | 3,665 | | | $ | 3,349 | |
| Finished goods | 26,323 | | | 24,509 | |
| Gross | 29,988 | | | 27,858 | |
| Excess and obsolete reserves | (868) | | | (532) | |
| $ | 29,120 | | | $ | 27,326 | |
| | | | | | | | | | | |
| 2023 | | 2022 |
Raw materials and supplies(a) | $ | 21,062 | | | $ | 18,454 | |
| Finished goods | 70,271 | | | 66,312 | |
| $ | 91,333 | | | $ | 84,766 | |
Changes in excess(a)Raw material inventory of $642 attributable to CartiHeal has been reclassified to current assets attributable to discontinued operations within the December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and obsolete reservesdivestitures and Note 15. Discontinued operations for inventory were as follows forfurther details regarding the years ended December 31:
| | | | | | | | | | | |
| 2020 | | 2019 |
| Balance, beginning of period | $ | (532) | | | $ | (570) | |
| Provision for losses | (904) | | | (870) | |
| Write-offs | 568 | | | 908 | |
| $ | (868) | | | $ | (532) | |
Property and equipment, net
Property and equipment consisted of the following as of December 31:
| | 2020 | | 2019 |
| 2023 | | | 2023 | | 2022 |
| Computer equipment and software | Computer equipment and software | $ | 20,547 | | | $ | 16,854 | |
| Demonstration and consignment inventory | |
| Leasehold improvements | Leasehold improvements | 3,126 | | | 2,918 | |
| Furniture and fixtures | Furniture and fixtures | 1,474 | | | 1,451 | |
| Machinery and equipment | 1,234 | | | 1,138 | |
| Finance leases | |
Machinery and equipment(a) | |
| Assets not yet placed in service | Assets not yet placed in service | 819 | | | 370 | |
| 27,200 | | | 22,731 | |
| 77,109 | |
| Less accumulated depreciation | Less accumulated depreciation | (20,321) | | | (18,242) | |
| $ | 6,879 | | | $ | 4,489 | |
| $ | |
(a)Machinery and equipment and accumulated depreciation attributable to CartiHeal of $269 and $78, respectively, were reclassified to long-term assets attributable to discontinued operations within the December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for further details regarding the deconsolidation of CartiHeal.
Depreciation expense from continuing operations was $2,106, $2,579$12,121, $5,688 and $3,439$3,204 for the years ended December 31, 2020, 20192023, 2022 and 2018,2021, respectively. The Company incurred a $2,038 disposal loss on fixed assets during the year ended December 31, 2023 as a result of the integration of acquisitions. The loss is recorded in loss on disposals within the consolidated condensed statements of operations and other comprehensive (loss) income.
Goodwill and intangible assets, net
There were no changes toChanges in the carrying amounts of goodwill by reportable segment during the years ended December 31, 20202023 and 2019. Following isDecember 31, 2022 are as follows:
| | | | | | | | | | | | | | | | | |
| U.S. | | International | | Consolidated |
| Balance at December 31, 2021 | $ | 138,863 | | | $ | 8,760 | | | $ | 147,623 | |
| Additions | 55,295 | | | 4,999 | | | 60,294 | |
| Deconsolidation of noncontrolling interest | (494) | | | — | | | (494) | |
| Purchase accounting adjustments | (4,467) | | | — | | | (4,467) | |
| Impairment | (189,197) | | | — | | | (189,197) | |
Reclassification(a) | — | | | (6,297) | | | (6,297) | |
| Balance at December 31, 2022 | $ | — | | | $ | 7,462 | | | $ | 7,462 | |
Balance at December 31, 2023(b) | $ | — | | | $ | 7,462 | | | $ | 7,462 | |
(a)Goodwill of $6,297 attributable to CartiHeal was reclassified to long-term assets attributable to discontinued operations within the December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for further details regarding the deconsolidation of CartiHeal.
(b)There was no activity related to goodwill during the year ended December 31, 2023.
Additions during the year ended December 31, 2022 were the result of the acquisition of CartiHeal and purchase accounting adjustments stem from changes in the preliminary fair values of assets acquired and liabilities assumed in previous and current acquisitions. Refer to Note 4. Acquisitions and divestitures for further information.
Due to the significant decline in the Company’s Class A common stock, the Company recorded a summarynon-cash goodwill impairment charge of $189,197 within the United States reporting unit for the year ended December 31, 2022 of which $124,697 was recorded in the impairment of assets and $64,500 in loss on discontinued operations, net of tax within the consolidated statements of operations and comprehensive (loss) income. The impairment was recorded within impairment of assets on the consolidated statements of operations and comprehensive (loss) income. There were no accumulated goodwill by reportable segment:impairment losses as of December 31, 2021.
| | | | | | | | | | | | | | | | | |
| U.S. | | International | | Consolidated |
| Balance at December 31, 2020 and 2019 | $ | 41,040 | | | $ | 8,760 | | | $ | 49,800 | |
Intangible assets, net
Intangible assets consisted of the following as of December 31:
| | 2020 | | 2019 |
| Intellectual property | $ | 263,422 | | | $ | 263,422 | |
| 2023 | | | 2023 | | 2022 |
Intellectual property(a)(b) | |
| Distribution rights | Distribution rights | 60,700 | | | 59,700 | |
| Customer relationships | Customer relationships | 57,700 | | | 57,700 | |
| IPR&D | IPR&D | 1,445 | | | 11,095 | |
| Developed technology and other | Developed technology and other | 13,999 | | | 4,649 | |
| Total carrying amount | Total carrying amount | 397,266 | | | 396,566 | |
| Less accumulated amortization: | Less accumulated amortization: | | | |
| Intellectual property | (117,281) | | | (100,982) | |
Intellectual property(a) | |
Intellectual property(a) | |
Intellectual property(a) | |
| Distribution rights | Distribution rights | (34,461) | | | (28,716) | |
| Customer relationships | Customer relationships | (51,247) | | | (46,407) | |
| Developed technology and other | Developed technology and other | (3,786) | | | (3,404) | |
| Total accumulated amortization | Total accumulated amortization | (206,775) | | | (179,509) | |
| Intangible assets, net before currency translation | Intangible assets, net before currency translation | 190,491 | | | 217,057 | |
| Currency translation | Currency translation | 1,159 | | | (547) | |
| $ | 191,650 | | | $ | 216,510 | |
| $ | |
(a)Intellectual property and accumulated depreciation attributable to CartiHeal totaling $410,200 and $11,327, respectively, were reclassified to long-term assets attributable to discontinued operations within the December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for further details regarding the deconsolidation of CartiHeal.
(b)The Company filed a 510(k) in 2019 and began commercializing a next-generation surgical productrecorded an impairment loss of $78,615 for the year ended December 31, 2023 in the third quarterU.S. reporting segment of 2020. As a result, $9,650net intellectual property attributable to the TheraSkin and TheraGenesis products, which were sold in May 2023. The loss was recorded in impairment of IPR&D was reclassified to developed technology and will be amortized over 10 years. On December 22, 2020, the Company entered into an amended and restated distribution agreement with the sole supplier of the Company’s five injection OA product. This agreement provided non-exclusive U.S. market distribution rights until December 31, 2028. The amended and restated distribution agreement created a $1,000 distribution right that will be amortized over 8 years, which was capitalized as an intangible asset and included in accrued liabilities onassets within the consolidated balance sheets asstatements of December 31, 2020.operations and comprehensive loss. Refer to Note 4. Acquisitions and divestitures for further details regarding businesses held for sale.
Amortization expense from continuing operations related to intangible assets was $27,565, $26,252$45,244, $55,715 and $26,622$35,480 for the years ended December 31, 2020, 20192023, 2022 and 20182021, respectively, of which $7,455, $6,416$23,848, $20,975 and $7,766$12,179 are included in ending inventory at December 31, 2020, 20192023, 2022 and 2018,2021, respectively. Estimated amortization expense for the years ended December 31, 20212024 through 20252028 is expected to be $28,262, $23,910, $22,297, $22,297$45,140, $42,503, $39,011, $38,659 and $21,259,$37,853, respectively.
Accrued liabilities
Accrued liabilities consisted of the following atas of December 31:
| | 2020 | | 2019 |
| 2023 | | | 2023 | | 2022 |
| Gross-to-net deductions | Gross-to-net deductions | $ | 43,656 | | | $ | 14,622 | |
| Bonus and commission | Bonus and commission | 15,188 | | | 14,200 | |
| Reserve for estimated overpayments from third-party payers | 2,790 | | | 6,801 | |
| Compensation and benefits | Compensation and benefits | 5,875 | | | 3,231 | |
| Accrued interest | |
| Income and other taxes | Income and other taxes | 2,434 | | | 2,555 | |
| Other liabilities | 18,244 | | | 11,418 | |
| $ | 88,187 | | | $ | 52,827 | |
Other liabilities(a) | |
| $ | |
(a)Other liabilities attributable to CartiHeal of $384 were reclassified into current liabilities attributable to discontinued operations within December 31, 2022 consolidated balance sheets. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for further details.
4. Business combinationsAcquisitions and investmentsdivestitures
VIEWound Business
On August 23, 2019,May 22, 2023, the Company purchased 285,714closed the sale of certain assets within its Wound Business, including the TheraSkin and TheraGenesis products (collectively, the “Wound Business” or the “Disposal Group”), for potential consideration of $84,675, including $34,675 at closing, $5,000 deferred for 18 months and up to $45,000 in potential earn-out payments (“Earn-out Payments”), which are based on the achievement of certain revenue thresholds by the purchaser of the Wound Business for sales of the TheraSkin and TheraGenesis products during the 2024, 2025 and 2026 fiscal years.
The Company incurred $3,880 in transactional fees resulting from the sale of the Wound Business. The loss resulting from the deconsolidation of the Disposal Group totaled $1,539 for the year ended December 31, 2023 and was recorded in loss on disposals within the consolidated statements of operations and comprehensive (loss) income. The Company used the proceeds from the sale of its Wound Business to prepay $30,000 of long-term debt obligations. Refer to Note 5. Financial Instruments for further details regarding the Company’s outstanding long-term debt obligations.
The Company evaluated the Wound Business for impairment prior to its sale and recorded a $78,615 impairment within the consolidated statements of operations and comprehensive (loss) income during the year ended December 31, 2023 as a result of this evaluation to reduce the intangible assets of the Disposal Group to reflect their respective fair values less any costs to sell. The fair value of the Disposal Group’s intangibles was determined based on the consideration received for the Wound Business.
CartiHeal (2009) Ltd.
On July 12, 2022, the Company completed the acquisition of 100% of the remaining shares in CartiHeal, a privately held company headquartered in Israel and the developer of Harbor’s Series C Preferred Stock or 3.1%the proprietary Agili-C implant for the treatment of joint surface lesions in traumatic and osteoarthritic joints. The Company previously held an equity interest in CartiHeal’s fully diluted shares with a carrying value of $15,768 and $16,771 as of July 12, 2022 and December 31, 2021, respectively. Net equity losses associated with CartiHeal for $1,000. the years ended December 31, 2022 and 2021 totaled $1,003 and $1,868, respectively, which are included in discontinued operations, net of tax on the consolidated statements of operations and comprehensive (loss) income.
The Company acquired CartiHeal (the “CartiHeal Acquisition”) for an aggregate purchase price of approximately $315,000 and Harboran additional $135,000, payable after closing upon the achievement of a certain sales milestone (“Sales Milestone”, or “CartiHeal Contingent Consideration”). The Company paid $100,000 of the aggregate purchase price upon closing consisting of a $50,000 deposit held in trust and $50,000 from a financing arrangement (Refer to Note 5. Financial instruments for further information regarding financing arrangements). The Company also paid approximately $8,622 of CartiHeal’s transaction-related fees and expenses and deferred $215,000 (“Deferred Amount”) of the aggregate purchase price otherwise due at closing.
The Deferred Amount was to be paid in five tranches commencing in 2023 and ending no later than 2027 as follows:
•$50,000 due upon the earliest to occur — the publication in a peer-reviewed orthopedic journal of an article that presents the results of the pivotal clinical trial (“First Paper Milestone”) or July 1, 2023;
•$50,000 due upon the earliest to occur — the implantation of Agili-C devices in 100 patients in the United States or September 1, 2023;
•$25,000 due upon the earliest to occur — the publication in a peer-reviewed orthopedic journal of an article that presents any new or additional clinical data subsequent to the First Paper Milestone with respect to Agili-C (“Second Paper Milestone”) or January 1, 2025;
•$25,000 due upon the earliest to occur — the publication in a peer-reviewed orthopedic journal of an article that presents any new or additional clinical data subsequent to the First and Second Paper Milestone with respect to Agili-C or January 1, 2026; and
•$65,000 due upon the earliest to occur — obtaining a U.S. Category 1 Current Procedural Terminology (“CPT”) code from Centers for Medicare and Medicaid Services (“CMS”) for Agili-C or January 1, 2027.
Pursuant to the CartiHeal Amendment (as defined below), the Company owed interest on each tranche of the Deferred Amount at a rate of 8.0% annually, until such tranche was paid. The Sales Milestone will be payable upon the achievement of $75,000 in trailing twelve month sales pursuant to the CartiHeal Amendment.
The Company had entered into an Option and Equity Purchase Agreement with CartiHeal (“Option Agreement”) in January 2020 and a subsequent amendment in June 2022 (“CartiHeal Amendment”). The Option Agreement provided the Company with an exclusive collaborationoption to acquire 100% of CartiHeal’s shares (“Call Option”), and provided CartiHeal with a put option that would require the Company to purchase 100% of CartiHeal’s shares under certain conditions. In August 2021, CartiHeal achieved pivotal clinical trial success, as defined in the Option Agreement, for the Agili-C implant. In order to preserve the Company’s Call Option, in accordance with the Option Agreement and upon approval of the Company’s Board of Directors (“BOD”), the Company deposited $50,000 into escrow in August 2021.
The First Paper Milestone under the Option Agreement occurred on February 13, 2023, which obligated the Company to make the first $50,000 payment, plus applicable interest, under the Option Agreement. On February 27, 2023, the Company entered into a settlement agreement (the “Settlement Agreement”) with Elron Ventures Ltd. (“Elron” and together with the Company, the “Parties”) as representative of CartiHeal’s selling securityholders under the Option Agreement (collectively, the “Former Securityholders”). Pursuant to the Settlement Agreement, Elron, on behalf of the Former Securityholders, agreed to forbear from initiating any legal action or proceedings relating to non-payment of any obligations arising under the Option Agreement during a period of 30 calendar days (the “Interim Period”) in exchange for purposes(i) a one-time non-refundable amount of developing$10,000 and (ii) a product for orthopedic usesone-time non-refundable payment of $150 to Elron to be commercializedused in accordance with the expense fund provisions of the Option Agreement. The Interim Period expired on March 29, 2023 and the Company did not exercise its right to extend the Interim Period. In addition, the Parties mutually released any further claims under the Option Agreement and related transaction documents, including without limitation a release by the Former Securityholders of any rights to enforce the provisions of the Option Agreement or make further monetary claims against the Company and/or its respective affiliates and supplied by Harbor. Asrepresentatives.
The Company transferred 100% of its shares in CartiHeal to a trustee (the “Trustee”) for the benefit of the Former Securityholders pursuant to the Settlement Agreement. The Company had no ownership interest and no voting rights during the Interim Period. Accordingly, the Company concluded that upon execution of the Settlement Agreement, the Company ceased to control CartiHeal for accounting purposes, and therefore, deconsolidated CartiHeal effective February 27, 2023. CartiHeal was part of the Company’s international reporting segment. The Company treated the deconsolidation of CartiHeal as a discontinued operation. The loss upon disposal was $60,639 and was recorded within discontinued operations, net of tax within the consolidated statements of operations and comprehensive (loss) income. The loss on disposal is comprised of the book value of CartiHeal’s net assets at the time of disposal, goodwill attributable to CartiHeal and the previously discussed non-refundable payments made to Elron. The Company allowed the Interim Period to expire on March 29, 2023 as the Company was not able to find a financing solution to fund the payment obligations under the Option and Equity Purchase Agreement on terms the Company believed to be favorable to it and its shareholders.
The fair value of consideration for the CartiHeal Acquisition is comprised of the following:
| | | | | |
| Cash consideration | $ | 100,000 | |
| Transaction related costs | 8,622 | |
| Subtotal of cash at closing | 108,622 | |
| Deferred Amount | 183,400 | |
| Sales Milestone | 61,901 | |
Fair value of previously held equity interest(a) | 39,477 | |
| Total consideration | $ | 393,400 | |
(a) Remeasurement of the Company’s equity method investment in CartiHeal, net of equity losses as a result of these transactions, the Company determined that it hadpurchase. The remeasurement included a variable interestgain of $23,709 calculated as the difference between the fair value and the carrying value of the Company’s investment in Harbor.CartiHeal at the acquisition date and was recognized in other income for the year ended December 31, 2023 on the consolidated statements of operations and comprehensive (loss) income. The fair value was based upon: (i) the consideration transferred to members owning 89.97% of CartiHeal’s fully diluted shares; (ii) calculating the value of CartiHeal’s fully diluted shares based upon the transferred consideration; and (iii) applying the calculated value to the Company’s 10.03% ownership in CartiHeal’s fully diluted shares at the acquisition date.
The Company accounted for the Harbor investment as a business combinationCartiHeal Acquisition using the acquisition method of accounting whereby the total purchase price was preliminarily allocated to tangible and intangible assets acquired and liabilities assumed based on respective fair values. The results of Harbor operations have been included in the accompanying consolidated financial statements subsequent to acquisition date. The Company did not disclose post-acquisition or pro forma losses attributable to Harbor as they did not have a material effect on the Company’s consolidated statements of operations and comprehensive income (loss).
The following table summarizes the fair values of the assets acquired and liabilities assumed at the acquisition date:
| | | | | |
| Fair value of consideration | $ | 393,400 | |
| Assets acquired and liabilities assumed: | |
| Cash and cash equivalents and restricted cash | $3,781 | | 1,430 |
Intellectual property (10-year useful life)Inventory | 4,834 642 | |
IPR&DPrepaid and other current assets | 1,445 552 | |
Other assetsProperty and equipment | 70 259 | |
| Intangibles | 410,200 | |
| Investment and other assets | 727 | |
Accounts payable and accrued liabilities | (932)(18) | |
| Accrued liabilities | (459) | |
| Other current liabilities | (1,696)(171) | |
| Deferred income taxes | (79,863) | |
| Other liabilities | (2,544) | |
| Net assets acquired | 333,106 | |
| Resulting goodwill | $ | 60,294 | |
Goodwill of $55,295 and $4,999 was allocated to the U.S. and International reporting units, respectively.
CartiHeal’s intangibles consists of the following:
| | | | | | | | | | | |
| Useful Life | | Fair Value |
Other long-term liabilitiesIntellectual property - US segment | (697)20 years | | $ | 351,500 | |
Deferred income taxIntellectual property - International segment | (266)8 years | | 58,700 | |
| Estimated fair value of net assets acquired | | $ | 4,188 410,200 | |
Bioventus purchase price | 1,000 | |
Fair value of Harbor’s noncontrolling interest | 3,188 | |
| $ | — | |
On March 27, 2020 two convertible promissory notesThe estimated fair value of the acquired CartiHeal intangibles was determined using an income approach, a valuation technique that estimates the fair value of an asset based on market participant expectations of the cash flows that an asset would generate over its remaining useful life. The determination of the useful lives was based upon consideration of market participant assumptions and transaction specific factors.
Misonix, Inc.
The Company acquired 100% of the capital stock of Misonix, Inc. (“Misonix”) in a cash-and-stock transaction (the “Misonix Acquisition”) on October 29, 2021. Total consideration for the Misonix Acquisition was $525,316 comprised of $274,562 in Bioventus Class A common stock, $223,118 in cash and $27,636 in value for Misonix options settled with Bioventus options. Misonix manufactures minimally invasive surgical ultrasonic medical devices used for precise bone sculpting, removal of soft and hard tumors and tissue debridement, primarily in the areas of neurosurgery, orthopedic surgery, plastic surgery and maxillo-facial surgery. Misonix also exclusively distributes skin allografts used to Harbor shareholders totaling $500 were converted into 142,858support healing.
Bioness, Inc.
The Company acquired 100% of the capital stock of Bioness, Inc. (“Bioness”) for $48,933 in cash and future contingent consideration payments (the “Bioness Acquisition”). Bioness is a global leader in neuromodulation and advanced rehabilitation medical devices through its innovative peripheral nerve stimulation therapy and premium advanced rehabilitation solutions.
Contingent consideration related to the Bioness Acquisition (“Bioness Contingent Consideration”) is comprised of future earn-out payments contingent upon the achievement of certain research and development projects as well as sales milestones related to Bioness products. The Bioness Acquisition Agreement includes maximum earn-out payments of $50,000 as follows: (i) $20,000 for meeting net sales targets for certain implantable products over a three year period ending on June 30, 2025 at the latest; (ii) up to $10,000 for meeting net sales milestones for certain implantable products over a three year period ending on June 30, 2025 at the latest; and (iii) $20,000 for maintaining Centers for Medicare & Medicaid Services coverage and reimbursement for certain products at specified levels as of December 31, 2024.
Investments
VIE
The Company had a fully diluted 8.8% ownership of Harbor Medtech Inc.’s (“Harbor”) Series C Preferred Stock and warrants for 428,572 sharesan exclusive Collaboration Agreement with Harbor, which resulted in the consolidation of Harbor. The Company terminated the Harbor common stock exercisable at a price of $1.167 per share with a 5-year exercise period expiring March 27, 2025. Promissory notes of $320 owed to a certain Harbor shareholder were converted into 92,500Collaboration Agreement on June 8, 2021, which resulted in the deconsolidation of Harbor Series C Preferred Stock at $3.50 per share in November 2020. On October 5, 2020,and the Company purchased an additional 285,714 sharesrecognition of a $5,674 impairment of Harbor’s Series C Preferred Stocklong-lived assets. The impairment was recorded within impairment of variable entity assets for $1,000. The Company continues to conclude that it is the primary beneficiary since it controls the significant activities of Harbor through the collaboration agreement. The noncontrolling interest related to Harbor was 91.2% as ofyear ended December 31, 2020.
The fair value of the Harbor intellectual property and IPR&D was determined using the income approach through an excess earnings analysis, with projected earnings discounted at a rate of 16.5%. The $1,445 of IPR&D consists of research and development progress toward developing a product for orthopedic uses. The fair value of the noncontrolling interest was calculated as estimated fair value of net assets acquired less the Company’s purchase price.
Harbor assets that can only be used to settle Harbor obligations and Harbor liabilities for which creditors do not have recourse to the general credit of the Company are as follows for the years ended December 31:
| | | | | | | | | | | |
| 2020 | | 2019 |
| Cash and cash equivalents | $ | 803 | | | $ | 1,127 | |
| Property and equipment, net | 173 | | | 60 | |
| Intangible assets, net | 5,635 | | | 6,122 | |
| Operating lease assets | 178 | | | 231 | |
| Other assets | 74 | | | 59 | |
| $ | 6,863 | | | $ | 7,599 | |
| | | |
| Accounts payable and accrued liabilities | $ | 366 | | | $ | 458 | |
| Other current liabilities | 2,004 | | | 2,395 | |
| Deferred income tax | — | | | 215 | |
| Other long-term liabilities | 659 | | | 872 | |
| $ | 3,029 | | | $ | 3,940 | |
Nearly all the liabilities assumed are payable to Harbor shareholders. As of December 31, 2019, other current liabilities primarily consisted of $1,8452022 in promissory notes to various Harbor shareholders and were scheduled to mature on August 31, 2020. These promissory notes were refinanced with additional borrowings in August 2020 and total $1,811 as of December 31, 2020. The Harbor promissory notes carry an 8% interest rate and are due on August 31, 2021 with payments due monthly.
Equity Method
Investments in which the Company can exercise significance influence, but do not control, are recorded under the equity method of accounting and are included in investments and other assets on the consolidated balance sheets. The Company’s share of net earnings or losses is included in other (income) loss within the consolidated statements of operations and comprehensive (loss) income, of which $5,176 was attributable to the non-controlling interest. An additional impairment of $1,369, representing the Company’s remaining investment balance in Harbor, was recorded within other (income) expense for the year ended December 31, 2021 in the consolidated statements of operations and comprehensive (loss) on a quarter lag.income. The Company evaluates investments for impairment whenever events or changes in circumstances indicate thatcontinues to have license rights to certain technology obtained from Harbor and is continuing product development initiated under the carrying amounts of such investments may be impaired. Impairment losses are recorded within earnings within the current period.Collaboration Agreement.
Other
On January 30, 2018,August 23, 2021, the Company purchased 337,397 shares of CartiHeal,Trice Medical, Inc.’s (“Trice”) Series D Preferred Stock for $10,000, representing an 8.4% ownership interest of its fully diluted shares. Trice is a privately held entity, Series F Convertible Preferred Stock or 2.8% of fully diluted sharescompany that develops and commercializes minimally invasive technologies for $2,500 in cash. The investment doessports medicine and orthopedic surgical procedures and it did not have a readily determinable fair value. On January 22, 2020,The investment in Trice was recorded at cost, less any impairment, plus or minus any changes resulting from observable price changes in orderly transactions for an identical or similar investment of the same issuer. In December 2022, the Company maderecognized an additional investment in CartiHeal, through a Simple Agreement for Future Equity (SAFE) by paying cashimpairment of $152. On July 15, 2020, CartiHeal completed an equity financing that the Company participated in and as a result, the Company received 12,825 in Series G-1 Preferred Shares and the SAFE terminated.
In addition, on July 15, 2020, the Company entered into an Option and Equity Purchase Agreement with CartiHeal. Under the terms of the agreement, the Company purchased 1,014,267 shares of CartiHeal Series G Preferred Shares for $15,000. As a result, the Company’s equity$10,285 representing its entire ownership in CartiHeal increasedinterest due to 10.03% of its fully diluted shares.Trice’s liquidity situation. The CartiHeal investment, included capitalized transaction costs of $1,427 and the $152 investment in January 2020, totaling $16,579impairment was recorded as an equity method investment beginning in July 2020, as the Company can exercise significant influence over CartiHeal but does not have control. It is included within investments and other assets(income) expense on the consolidated balance sheet. The CartiHeal investment carrying value is $18,689 asstatements of operations and comprehensive (loss) income for the year ended December 31, 2020, after recording net losses of $467 incurred during 2020.2022.
The Company will, if needed, purchase an additional 338,089 of CartiHeal Series G Preferred Shares for $5,000, for the completion of a certain study. The Company has an exclusive option to acquire the remaining equity in CartiHeal, which may be exercised at any time up to and within 45 days following notice of the U.S. Food and Drug Administration (FDA) approval for a CartiHeal product currently in development. In addition, upon the same FDA approval, CartiHeal may exercise an option within 45 days that requires the Company to complete the acquisition of the remaining equity in CartiHeal.
5. Financial instruments
2019 Credit AgreementLong-term debt consisted of the following as of December 31:
| | | | | | | | | | | |
| 2023 | | 2022 |
| Amended Term Loan due October 2026 (9.89% at December 31, 2023) | $ | 382,448 | | | $ | 420,712 | |
| Revolver due October 2025 (9.88% at December 31, 2023) | 15,000 | | | — | |
| Less: | | | |
| Current portion of long-term debt | (27,848) | | | (33,056) | |
| Unamortized debt issuance cost | (917) | | | (1,338) | |
| Unamortized discount | (1,685) | | | (1,308) | |
| $ | 366,998 | | | $ | 385,010 | |
Amended Term Loan
On December 6, 2019, the Company entered into a $250,000 creditCredit and guaranty agreement (2019Guaranty Agreement (the “2019 Credit Agreement) with Wells Fargo Bank National Association (Wells), as well as a syndicate of other banks, or Lenders. The 2019 Credit Agreement isAgreement”) that was comprised of a $200,000 term loan (Term Loan), with an original issue discount (OID) of $666,(“Original Term Loan”) and a $50,000 revolving facility (Revolver)(the “Revolver”). The Company amended the 2019 Credit Agreement on August 29, 2021, and then again on October 29, 2021 in connection with the Misonix Acquisition in which the Company prepaid $80,000 on the Original Term Loan. The 2019 Credit Agreement, as amended, subsequent to the prepayment, was comprised of a $360,750 term loan (“Term Loan”) and the Revolver.
On July 11, 2022, the Company further amended the 2019 Credit Agreement in conjunction with the CartiHeal Acquisition. Pursuant to that amendment, an $80,000 term loan facility (the “July 2022 Term Loan” and, together with the Term Loan, the “Term Loan Facilities”) was extended to the Company to be used for: (i) the financing of the CartiHeal Acquisition; (ii) the payment of related fees and expenses; (iii) repayment of the draws made on the Revolver; and (iv) working capital needs and general corporate purposes of the Company, including without limitation for permitted acquisitions.
The Company was not in compliance with certain financial covenants as of December 31, 2022. As a result, on March 31, 2023, the Company entered into another amendment to the 2019 Credit Agreement to, among other things, modify certain financial covenants, waive the noncompliance at December 31, 2022, and to modify interest rates applicable to borrowings under the 2019 Credit Agreement. The Company was in compliance as of December 31, 2023 with the financial covenants as stated within the 2019 Credit Agreement then in effect.
On January 18, 2024 (the “Closing Date”), the Company further amended the 2019 Credit Agreement (collectively, with the August 2021, October 2021, July 2022 and March 2023 amendments, the “Amended 2019 Credit Agreement”), to further modify certain financial covenants under the 2019 Credit Agreement.
The Term Loan Facilities will now mature on October 29, 2026 (“Maturity”). The Revolver will mature on October 29, 2025.
SOFR loans and base rate loans had a margin of 3.25% and 2.25%, respectively, subsequent to July 11, 2022 and prior to the Closing Date. As of the March 31, 2023 amendment, SOFR loans and base rate loans had a margin of 4.25% and 3.25%, respectively. All obligations under the Amended 2019 Credit Agreement are guaranteed by the Company and certain of the Company’s wholly owned subsidiaries. Substantiallysubsidiaries where substantially all the assets of the Company collateralize the obligations under theobligations.
The Amended 2019 Credit Agreement. Agreement contains customary affirmative and negative covenants, including those related to financial reporting and notification, restrictions on the declaration or payment of certain distributions on or in respect of Bioventus LLC’s equity interests, restrictions on acquisitions, investments and certain other payments, limitations on the incurrence of new indebtedness, limitations on transfers, sales and other dispositions of assets of Bioventus LLC and its subsidiaries, as well as limitations on making changes to the business and organizational documents of Bioventus LLC and its subsidiaries. Financial covenant requirements include (i) a maximum debt leverage ratio of not greater than 5.65 to 1.00 for the testing period ending March 31, 2024, 5.00 to 1.00 for the testing period ending June 30, 2024, 4.75 to 1.00 for the testing period ending September 30, 2024, 4.50 to 1.00 for the testing period ending December 31, 2024, 4.25 to 1.00 for testing period ending March 31, 2025, 4.00 to 1.00 for testing period ending June 30, 2025 and each testing period thereafter, and, beginning with the testing period ending March 31, 2025, to be subject to a temporary increase to 4.50 to 1.00 upon certain events, and (ii) an interest coverage ratio not less than 1.75 to 1.00 for the testing period ending March 31, 2024, 1.75 to 1.00 for the testing period ending June 30, 2024, 1.75 to 1.00 for the testing period ending September 30, 2024, 2.00 to 1.00 for the testing period ending December 31, 2024, 2.00 to 1.00 for the testing period ending March 31, 2025, 2.25 to 1.00 for the testing period ending June 30, 2025, 2.50 to 1.00 for the testing period ending September 30, 2025 and 3.00 to 1.00 for the testing period ending December 31, 2025 and each testing period thereafter. In addition, during the period commencing on the Closing Date and ending upon the satisfaction of certain conditions occurring not prior to October 29, 2025, the Company will be subject to certain additional requirements and covenants, including a requirement to maintain Liquidity (as defined in the Amended 2019 Credit Agreement) of not less than $10,000 as of the end of each calendar month during such period.
The July 2022 Term Loan had an original issue discount of $240 and deferred financing costs of $101. No financing or deferred costs were expensed and there was no loss on debt refinancing and modification as a result of the July 2022 amendment. The Company paid financing costs of $3,318 as a result of the October 2021 amendment, of which $1,421 was capitalized to the consolidated balance sheets and $1,897 was recorded in selling, general and administrative expenses during the year ended December 31, 2021 within the consolidated statements of operations and comprehensive (loss) income. Due to the change in participating lenders, an additional $269 in deferred costs was recorded in interest expense for the year ended December 31, 2021. Loss on debt refinancing and modification totaled $2,162 for the year ended December 31, 2021. Capitalized deferred fees from the October 2021 amendment totaled $3,174 and $893 for the Term Loan and the Revolver, mature on December 6, 2024 (Maturity).
Term Loanrespectively.
As of December 31, 2020, $188,3782023, $379,846 was outstanding on the Term Loan Facilities, net of original issue discount of $524$1,685 and deferred financing costs of $1,098. As$917. Capitalized deferred fees are amortized to interest expense on a straight-line basis over the term of December 31, 2020, the Term Loan Facilities, which approximates the effective interest rate including a margin of 2.25% was 2.40%.method. The Company recorded $1,706, $853 and $588 for deferred cost amortization in interest expense for the years ended December 31, 2023, 2022 and 2021, respectively. Scheduled quarterly principal payments of the Term Loan Facilities are as follows$3,056 for the first quarter of 2024, $8,264 for the second, third and fourth quarters of 2024 and $11,019 for the year 2025 and the first three quarters of 2026 with thea final payment of $125,000$277,469 at Maturity:
| | | | | |
| Quarterly
payment
|
2021 and 2022 | $ | 3,750 | |
2023 and 2024 | $ | 5,000 | |
Maturity. Contractual maturities of long term debt for the next three years are as follows: 2024—$27,848, 2025—$44,075 and 2026—$310,525, respectively.The Company may voluntarily prepay the Term Loan Facilities without premium or penalty upon prior notice. The Company may be required to make additional principal payments on the Term Loan Facilities dependent upon the generation of certain cash flow events as defined in the Amended 2019 Credit Agreement. These additional prepayments will be applied to the scheduled installments of principal in direct order of maturity of first the Base Rate (BR) portions of the Term Loan firstFacilities and then the Eurodollar portions of the Term Loan.portions.
The estimated fair value of the Term Loan Facilities was $382,926 as ofDecember 31, 2020 was $189,534.2023. The fair value of these obligations was determined using a discounted cash flow model based on current market interest rates available to the Company. These inputs are corroborated by observable market data for similar obligations and aremidpoint of the Bloomberg Valuation, as of December 31, 2023. This is classified as a Level 2 instruments within the fair value hierarchy.
Revolver
The Revolver is a five-year revolving credit facility of $50,000 whichthat includes revolving and swingline loans as well as letters of credit (LOC)(“LOC”) and, inclusive of all, cannot exceed $50,000$45,000 at any one time.time as the Revolver capacity was reduced at December 31, 2023 in accordance with the 2019 Credit Agreement then in effect. The Revolver credit capacity will be further reduced by $5,000 on June 30, 2024. LOCs are available in an amount not to exceed $7,500. Revolving loans are due at the earlier of termination or Maturity. Swingline loans are available as BR interest rate option loans only and must be outstanding for at least five days. Swingline loans are due the fifteenth or last day of a calendar month or Maturity whichever is earlier. The Company had increased its cash position by borrowing $49,000 on its Revolver as a precautionary measure to preserve financial flexibility in view of the current uncertainty resulting from the COVID-19 pandemic during the first quarter of 2020. The $49,000 was repaid during the third quarter of 2020. As of December 31, 2020, there was one nominal LOC2023, the Company had three LOCs outstanding leaving approximately $49,912$43,200 available. The Revolver had $15,000 in outstanding borrowings as of December 31, 2023 and zero at December 31, 2022.
Interest
The Term Loan Facilities and Revolver permitspermit the Company to elect either Eurodollarthe secured overnight financial rate (“SOFR”) or BRbase interest rate (“BR”) options for the entire amount or certain portions of the loansloans. Both the SOFR and BR options have interest rates equal to a formula driven base interest rate plus a margin, tied to a leverage ratio. The leverage ratio is the ratio of debt to consolidated EBITDA as defined in the Amended 2019 Credit Agreement, or Bank EBITDA, for four consecutive quarters at the end of each period.
Agreement. BR portions of the Term Loan Facilities have interest due the last day of each calendar quarter-end. EurodollarPursuant to the Amended 2019 Credit Agreement, the margin at each applicable leverage ratio will be increased by 1.00% per annum. SOFR portions of the Term Loan Facilities have one, two, three or six-month interest reset periods and interest is due on the last day of each three-month period or the last day of the loan term if less than three months. In advance of the last day of the current Eurodollar Loan, the Company may select a new loan type so long as it does not extend beyond Maturity. The outstanding Term Loan has been a Eurodollar Loan since inception and is an auto-renewing one-month loan for setting an interest rate. In addition, the Term Loan has an interest due date concurrent with any scheduled principal repayment or prepayment.
Interest is calculated based on a 360-day year except for BR loans where the base interest is the Wells Prime Rate, in which case it is calculated based on a calendar-day year. The base interest rate for all BR loans is equal to the highest of (a) the Wells Prime Rate, (b) the greater of the Federal Funds Effective Rate or Overnight Bank Funding Rate plus 1/2% and (c) the Eurodollar Rate for a USD deposit with a maturity of one month plus 1.0%. The base interest rate for all Eurodollar Loans is equal to the rate determined for such day in accordance with the following formula with the Term Loan having a floor of 0%:
| | | | | | | | |
| LIBOR | |
1—Eurocurrency Reserve Requirements |
Pricing grids are used to determine the applicable loan margins based on the type of loan and the leverage ratio. The initial Eurodollar and BR loans had a margin of 2.25% and 1.25%, respectively. Loanloan margin is adjusted after the quarterly financial statements are delivered to the lenders in accordance with the below pricing grid, below:which reflects the margins in effect under the Amended 2019 Credit Agreement:
| | | | | | | | | | | | | | |
| Leverage ratio | | Eurodollar | | BR |
| > 2.50 to 1.00 | | 2.50 | % | | 1.50 | % |
| >1.50 to 1.00 and < 2.50 to 1.00 | | 2.25 | % | | 1.25 | % |
| > 1.25 to 1.00 and <1.50 to 1.00 | | 1.75 | % | | 0.75 | % |
| > 0.75 to 1.00 and <1.25 to 1.00 | | 1.50 | % | | 0.50 | % |
| < 0.75 to 1.00 | | 1.25 | % | | 0.25 | % |
| | | | | | | | | | | |
| Leverage ratio | SOFR | | BR |
| > 4.00 to 1.00 | 4.25 | % | | 3.25 | % |
| ≥ 3.50 to 1.00 and < 4.00 to 1.00 | 3.75 | % | | 2.75 | % |
| ≥ 3.00 to 1.00 and < 3.50 to 1.00 | 3.25 | % | | 2.25 | % |
| ≥ 2.50 to 1.00 and < 3.00 to 1.00 | 3.00 | % | | 2.00 | % |
| < 2.50 to 1.00 | 2.75 | % | | 1.75 | % |
The Revolver includes a commitment fee at 0.25%0.30% of the average daily amount of the available revolving commitment, assuming any swingline loans outstanding are $0.zero. There were no swingline loans outstanding as of December 31, 2020.2023. The fee is payable quarterly in arrears on the last day of the calendar quarters and at Maturity. The commitment fee rate is adjusted after the quarterly financial statements are delivered to lenders based on the below pricing grid below:under the Amended 2019 Credit Agreement:
| | | | | | | | |
| Leverage ratio | | Commitment fee rateFee Rate
|
>≥ 2.50 to 1.00 | 0.30 | 0.30 | % |
>1.50 to 1.00 and < 2.50 to 1.00 | 0.20 | 0.25 | % |
> 1.25 to 1.00 and <1.50 to 1.00 | | 0.20 | % |
> 0.75 to 1.00 and <1.25 to 1.00 | | 0.15 | % |
< 0.75 to 1.00 | | 0.10 | % |
Fees are charged on all outstanding LOCs at an annual rate equal to the margin in effect on Eurodollar revolving loans. A frontingfunding fee of 0.125% per year on the undrawn and unexpired amount of each LOC is payable as well. The fees are payable quarterly in arrears on the last day of the calendar quarters.
As of December 31, 2020, the The Company’s effective weighted average interest rate onwas 9.92% for all outstanding debt, including the commitment fee and interest rate swap, was 2.72%.
Other
The 2019 Credit Agreement contains customary affirmative and negative covenants, including those related to financial reporting and notification, restrictions on the declaration or payment of certain distributions on or in respect of the Company’s equity interests, restrictions on acquisitions, investments and certain other payments, limitations on the incurrence of new indebtedness, limitations on transfers, sales and other dispositions of Company assets, as well as limitations on making changes to the Company’s business and organizational documents. Financial covenant requirements include a maximum debt leverage ratio as well as an interest coverage ratio not less than 3.00 to 1.00 as defined in the 2019 Credit Agreement. As of December 31, 2020, the Company complied with the financial covenants in the 2019 Credit Agreement.
Each Lender may provide an additional Term or Revolving Loan by executing and delivering notice specifying the terms, if doing so would not cause certain undesired events to occur as defined in the 2019 Credit Agreement or extend repayment beyond Maturity. The aggregate amount of all additional borrowings may not exceed the greater of $100,000 and the trailing four quarters Bank EBITDA without the consent of the Lenders holding more than 50% of the total outstanding debt under the 2019 Credit Agreement.
Financing costs
During December 2019, the Company paid financing costs totaling $2,117 in order to refinance our prior term loan facility, that was repaid in full (Prior Credit Agreement). The Company recorded $269 directly to selling, general and administrative expense and the remaining $1,848 was capitalized to the consolidated balance sheet. One lender participating in the Prior Credit Agreement became a lender in the 2019 Credit Agreement and, as a result, $2,985 related to the Prior Credit Agreement was written off and recorded as interest expense. The $269 recorded in selling, general and administrative expense and the $2,985 recorded in interest expense total the $3,252 of loss on debt retirement and modification.
Total capitalized deferred fees for the Term Loan of $1,398 and Revolver of $653 are being amortized to interest expense on a straight-line basis over each of the respective lives, which approximates the effective interest method. The Company recorded $543, $711 and $745 in interest expense associated with these deferred costs for the years ended December 31, 2020, 2019 and 2018, respectively.
Contractual maturities of long-term debt as of December 31, 2020, were as follows:2023. Cash paid for interest totaled $32,470, $18,043 and $5,837 for the December 31, 2023, 2022 and 2021, respectively.
| | | | | | | | |
| 2021 | | $ | 15,000 | |
| 2022 | | 15,000 | |
| 2023 | | 20,000 | |
| 2024 | | 140,000 | |
| 2025 and thereafter | | — | |
| Deferred financing costs | | (1,098) | |
| Original issue discount | | (524) | |
| Total long-term debt | | 188,378 | |
| Less current portion | | (15,000) | |
| Total | | $ | 173,378 | |
The Company entershistorically entered into interest rate swap agreements to limit its exposure to changes in the variable interest rate on its long-term debt. On March 26, 2020, theThe Company entered anhad one non-designated interest rate swap agreement with one ofthat was terminated on October 28, 2022 and subsequently received $7,738 upon its Lenders, which expires in December 2024.termination. The interest rate swap was not designated as a hedge. The Company has no other active derivatives and the swap is carried at fair value on the balance sheet. Refer to Note 7. Fairsheet with changes in fair value measurements for further details regarding the Company’srecorded as interest rate swap. There were no outstanding derivatives as of December 31, 2019. Interestincome or expense of $1,599 was recorded within the consolidated statements of operations and comprehensive (loss) income. Net interest income (loss)of $6,396 and $2,730 was recorded related to the change in fair value of the interest rate swap for the yearyears ended December 31, 2020.
The notional value of the swap totaled $100,000 or 52.6%, of the Term Loan outstanding principal at December 31, 2020. The swap locked in the variable portion of the interest rate on the $100,000 notional at 0.64%, with a stated fixed rate of 2.25%. The effective interest rate of the swap was 2.89% as of December 31, 2020.
6. Members’ equity
Members’ equity consisted of the following at December 31:
| | | | | | | | | | | |
| 2020 | | 2019 |
| Preferred | $ | 168,000 | | | $ | 168,000 | |
| Common | 113,373 | | | 113,373 | |
Profits interest and other equity awards (Refer to Note 9. Equity-based compensation) | 3,800 | | | 3,774 | |
| $ | 285,173 | | | $ | 285,147 | |
The authorized number of common2022 and preferred units were unlimited. On May 4, 2012, 4,900,000 common units and 5,100,000 preferred units (2012 Preferred Units) were issued. During November 2015, the Company obtained a $50,000 capital contribution from its existing members and 1,490,000 in preferred units were issued (2015 Preferred Units). The common and preferred members have stated rights and privileges, which include, but are not limited to: (1) voting and Company governance, (2) the transfer of membership interests and (3) dissolution and liquidation of the Company.2021, respectively.
Each preferred unit carried a priority payout (Liquidation Preference), as defined in the Company’s limited liability company agreement in effect at December 31, 2020. The initial Liquidation Preference for the 2012 and 2015 Preferred Units were $23.14 and $33.57, respectively. The preferred units accrued a distribution right at a rate of 3% per annum and was added annually to the Liquidation Preference. On February 11, 2021, the common and preferred units, including the preferred distribution rights, were converted into Common LLC Interests as discussed in Note 1. Organization and basis of presentation of financial information and no further distribution rights accrued.
The Continuing LLC Owner owned the only Equity Participation Right Unit (EPR Unit). The EPR Unit was junior to the common units and its only entitlement was 0.55% of available distributions arising from the closing of a sale of units representing a percentage interest of more than 66.66%, or the sale of all or substantially all of the assets of the Company, provided such event constitutes a change of control (Distribution Event) as described in Note 7. Fair value measurements. In February 2021, the EPR Unit was redeemed in exchange for $3,327 in connection with the IPO at which time the EPR ceased to exist and all entitlements ended. Refer to Note 17. Subsequent events for further details regarding the impact of the IPO on members’ equity.
7.6. Fair value measurements
As of December 31, 2020, thereThere were no assets measured at fair value on a recurring basis and there were no liabilities measuredvalued at fair value using Level 1 inputs.inputs at December 31, 2023 and 2022. The following table provides information for assets and liabilities measured at fair value on a recurring basis using Level 2 and Level 3 inputs.inputs:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 | | December 31, 2019 |
| Total | | Level 2 | | Level 3 | | Level 3 |
| Interest rate swap | $ | 1,602 | | | $ | 1,602 | | | $ | — | | | $ | — | |
| Management incentive plan and liability-classified awards | 40,303 | | | — | | | 40,303 | | | 40,802 | |
| Equity Participation Rights | 6,101 | | | — | | | 6,101 | | | 5,457 | |
| Total liabilities | $ | 48,006 | | | $ | 1,602 | | | $ | 46,404 | | | $ | 46,259 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 | | December 31, 2022 |
| Total | | Level 2 | | Level 3 | | Total | | Level 2 | | Level 3 |
| Liabilities: | | | | | | | | | | | |
Deferred Amount - Current(a) | $ | — | | | $ | — | | | $ | — | | | $ | 117,615 | | | $ | — | | | $ | 117,615 | |
Deferred Amount - Long Term(a) | — | | | — | | | — | | | 79,269 | | | — | | | 79,269 | |
CartiHeal Contingent Consideration(a) | — | | | — | | | — | | | 67,251 | | | — | | | 67,251 | |
| Bioness Contingent Consideration | 18,150 | | | — | | | 18,150 | | | 17,431 | | | — | | | 17,431 | |
| Total liabilities: | $ | 18,150 | | | $ | — | | | $ | 18,150 | | | $ | 281,566 | | | $ | — | | | $ | 281,566 | |
Interest rate swap(a)The Deferred Amount and contingent consideration attributable to CartiHeal have been reclassified to discontinued operations within the December 31, 2022 balance sheet. CartiHeal was fully deconsolidated during the first quarter of 2023. Refer to Note 4. Acquisitions and divestitures and Note 15. Discontinued operations for further details regarding the deconsolidation of CartiHeal.
Deferred Amount
The Deferred Amount that resulted from the CartiHeal Acquisition was calculated based on the total amount payable on each due date for the payment tranches including applicable interest. As previously discussed, the Company reached a Settlement Agreement with the Former Securityholders which relieved the Company of the obligations under the Deferred Amount. Refer to Note 4. Acquisitions and divestitures for further information.
Contingent consideration
The Company initially values interest rate swapscontingent consideration related to business combinations using discounted cash flows. Forward curves and volatility levels are used to estimate future cash flows that are not certain. These are determined using observable market inputs when available and based on estimates when not available. The fair valuea probability-weighted calculation of the swap was recorded in the Company’s consolidated balance sheets within accrued liabilities. Changes in fair value are recognized as interest expense within the consolidated statements of operations and comprehensive income (loss).
Management incentive plan and liability-classified awards
Prior to the IPO, the Company had operated two-equity-based compensation plans, the Management Incentive Plan (MIP) and the Phantom Profits Interest Plan (Phantom Plan). The estimated fair value reflects assumptions made by management as of December 31, 2020, including the impact of COVID-19 on significant unobservable assumptions, such as the expected timing and volume of elective procedures and the impact of these procedures on future revenues which impact the equity value. However, the actual amount ultimately paid could be higher or lower than the fair value. The Company has classified $11,054 as accrued equity-based compensation and $29,249 as accrued equity-based compensation, less current portion as of December 31, 2020. Any changes in fair value are recorded as an operating expense and included within selling, general administrative expense and research and development expense on the consolidated statement of operations and comprehensive income (loss) based upon the classification of the employee.
The following table provides a reconciliation of the beginning and ending balances for the MIP and liability-classified awards at fair value using significant unobservable inputs or Level 3:
| | | | | | | | |
Balance at December 31, 2018 | | $ | 33,063 | |
Initial estimate (vesting) | | 5,464 | |
Forfeitures | | (1,013) | |
Change in fair value | | 6,290 | |
Payment | | (3,002) | |
Balance at December 31, 2019 | | 40,802 | |
Initial estimate (vesting) | | 4,734 | |
Forfeitures | | (1,298) | |
Change in fair value | | 6,641 | |
Payment | | (10,576) | |
Balance at December 31, 2020 | | $ | 40,303 | |
In June 2020, the sole MIP awardee exercised the right to force a cash settlement for 150,252 of the 333,330 vested units resulting in a payment of $6,329. The remaining 183,078 units were settled for $10,802 in February 2021.
EPR Unit
Prior to the IPO, after which the EPR ceased to exist and all entitlements ended, the Continuing LLC owners owned the only EPR Unit and its only entitlement was 0.55% of available distributions arising from a Distribution Event. The estimated fair value reflects assumptions made by management as of December 31, 2020, including potential payment scenarios discounted at rates reflective of the risks associated with the expected future cash flows. The fair valueflows for certain milestones. For other milestones, the Company used a variation of the EPR Unitincome approach where revenue was recordedsimulated in the Company’s consolidated balance sheets as other long-term liabilities. The revaluation for the EPR liability is recognized in interest expense on the consolidated statements of operations and comprehensive income (loss).a risk-neutral framework using Geometric Brownian Motion, a stock price behavior model.
The following table provides a reconciliation of the beginning and ending balances for the EPR Unit at fair value using significant unobservable inputs Level 3:
| | | | | | | | |
Balance at December 31, 2018 | | $ | 4,892 | |
Change in fair value | | 565 | |
Balance at December 31, 2019 | | 5,457 | |
Change in fair value | | 644 | |
Balance at December 31, 2020 | | $ | 6,101 | |
The Company estimatedKey assumptions used to estimate the fair value of contingent consideration include projected financial information, market data and the Plansprobability and EPR Unittiming of achieving the specific targets. After the initial valuation, the Company generally uses its best estimate to measure contingent consideration at each subsequent reporting period using a Monte Carlo simulation. This fair value measurement is based on significant inputs that are unobservable in the market, and thus represents a Level 3 measurement. The key assumptions used in applyinginputs. As previously discussed, the valuation model includeCompany reached a Settlement Agreement with CartiHeal’s selling securityholders. As a result of the Company’s equity value,Settlement Agreement, the expected timing until a liquidity event, applicable discount rates applied,Company was relieved of the CartiHeal Contingent Consideration obligations. Refer to Note 4. Acquisitions and equity volatility. In addition,divestitures for further information.
Unobservable inputs
A summary of unobservable Level 3 inputs utilized for the EPR Unit, the estimated accrued preferred distribution at the liquidity event date totaling $43,854above liabilities are as of December 31, 2020 as it is senior in order of payment. follows:
| | | | | | | | | | | | | | | | | |
| Valuation Technique | | Unobservable inputs | | Range |
| Bioness Contingent Consideration | Discounted cash flow | | Payment discount rate | | 6.4% - 6.8% |
| | | Payment period | | 2024 - 2025 |
Significant changes in these assumptions could result in a significantly higher or lower fair value.
The followingcontingent consideration reported in the above table provides a rangeresulted from the Bioness Acquisition on March 30, 2021 and the CartiHeal Acquisition on July 12, 2022. Contingent consideration is adjusted quarterly based upon the passage of key assumptions used withintime or the valuationanticipated success or failure of the awards as of December 31, 2020:
| | | | | | | | | | | | | | | | | | | | |
| Valuation technique | | Unobservable inputs | | Range | | Weighted Average |
| Option pricing approach | | Time to liquidity event | | 0.4 | | 0.4 |
| | Risk free rate | | 0.10% | | 0.10% |
| | Equality volatility | | 35.13% - 101.25% | | 50.0% |
| | Equity value | | $1,065,000 - $1,240,000 | | $1,145,000 |
| | Lack of marketability discount | | 7.0% | | 7.0% |
8. Restructuring costs
Restructuring costs are not allocatedachieving certain milestones. Changes in contingent consideration related to the Company’s reportable segments as they are not part of the segment performance measures regularly reviewed by management. These charges are included in restructuring expenses in the consolidated statement of operationsBioness Acquisition totaled $719, $1,102 and comprehensive income (loss).
In the fourth quarter of 2020 and 2018, the Company adopted restructuring plans to improve the performance of International operations, principally through headcount reduction and closing offices in certain countries as the Company shifts to an indirect distribution model in these countries. The plans were completed in 2020 and 2019, respectively, and the Company recorded total pre-tax charges of $563, $575 and $1,373 primarily related to severance$829 for the years ended December 31, 2020, 20192023, 2022 and 2018, respectively.2021, respectively, and were recorded as the change in fair value of contingent consideration within the consolidated statements of operations and comprehensive (loss) income. Changes in contingent consideration related to the CartiHeal Acquisition totaled $1,710 and $5,350 for years ended December 31, 2023 and 2022, respectively and were recorded in discontinued operations, net of tax within the consolidated statements of operations and comprehensive (loss) income. comprehensive loss. Pursuant to the Settlement Agreement, the Company was relieved of CartiHeal related obligations. The Company’s costs totaled $563Company deconsolidated the remaining $68,961 contingent consideration liability as a result. Refer to Note 4. Acquisitions and divestitures for further details regarding the 2020deconsolidation of CartiHeal.
Management incentive plan and $1,948 for the 2018 plan.liability-classified awards
The Company’s restructuring charges and payments for all plans are comprised of the following:
| | | | | | | | | | | | | | | | | |
| Employee severance and temporary labor costs | | Other charges | | Total |
| Balance at December 31, 2018 | $ | 997 | | | $ | 206 | | | $ | 1,203 | |
| Expenses incurred | 491 | | | 84 | | | 575 | |
| Payments made | (1,488) | | | (290) | | | (1,778) | |
| Balance at December 31, 2019 | — | | | — | | | — | |
| Expenses incurred | 408 | | | 155 | | | 563 | |
| Payments made | (242) | | | (74) | | | (316) | |
| Balance at December 31, 2020 | $ | 166 | | | $ | 81 | | | $ | 247 | |
9. Equity-based compensation
Equity-based compensation plans
The CompanyBV LLC had operated two equity-based compensation plans, the MIPmanagement incentive plan (the “MIP”) and the BV LLC Phantom Profits Interest Plan (the Plans) prior to(together with the IPO. The awards granted under both plans represented a non-managing, non-voting interestMIP, the “Phantom Plans”), which were terminated on February 11, 2021 in connection with the Company designed for grantees to share in the future appreciation of the value of the Company.Company’s IPO. Awards granted under the MIP Plan and the 2015 Phantom Units arewere liability-classified and the 2012 Phantom Units arewere equity-classified. On February 11,Prior to the IPO and during the year ended December 31, 2021, in conjunctionthe Company settled with the IPO, the Plans were terminated and there were no furthersole MIP awardee for $10,802. No awards under the Plans. As a result,Phantom Plans were granted post-IPO and the New LLC Owner assumed the obligations of the Company’s Phantom Plan awards on February 10,were settled 12 months following the termination. Vested awardees whose BV LLC employment terminated prior to the IPO had their awards settled in March 2022 for $10,413. Due to the changes in fair value for these awards granted prior to the Company’s IPO, compensation award activity from the Phantom Plans resulted in a net gain of $24,356 for the year ended December 31, 2021.
The awards
7. Equity-based compensation
Terminated plans
Prior to the Phantom Plans’ termination, during the year ended December 31, 2021, (i) the Company granted under the MIP fully vested at December 2, 2017. There90,000 Phantom Profits Interest Plan units; (ii) there were no MIP awards granted; (iii) 900 Phantom Profits Interest Plan units were forfeited; and (iv) other Phantom Plan units were redeemed for $479. Compensation expense related to the Phantom Plans totaled $829 for the year ended December 31, 2021. This amount excludes the $25,185 decrease in fair market value of accrued equity-based compensation due to adjustments to reflect the difference between the expected public offering price of the IPO and the actual offering price, of which $1,777 was recorded in research and development expense within the consolidated statements of operations and comprehensive (loss) income for the year ended December 31, 2021.
2021 Plan
The Company operates an equity-based compensation plan (“2021 Plan”), which allows for the issuance of stock options (incentive and nonqualified), restricted stock, dividend equivalents, restricted stock units (“RSUs”), other stock-based awards, and cash awards (collectively, “Awards”). As of December 31, 2023, 14,781,895 shares of Class A common stock were authorized to be awarded and 7,843,854 shares were available for 2021 Plan Awards.
2023 Plan
The Company also operates the 2023 Retention Equity Award Plan (the “2023 Plan” and, together with the 2021 Plan, the “Plans”), the purpose of which is to retain and motivate critical personnel over the short-term by providing them additional incentives in the form of RSUs (the “Retention Awards” and together with the “2021 Plan Awards,” the “Awards”). As of December 31, 2023, 600,000 shares of Class A common stock were authorized to be awarded under the 2023 Plan and 73,200 shares were available for Retention Awards.
Activity under the Plans
Expense
Equity-based compensation expense for Awards granted under the Plans and inducement awards for the years ended December 31, 2020, 20192023, 2022 and 2018. In June 2020, the sole MIP awardee exercised the right to force a cash settlement for 150,252 of the 333,330 vested units resulting in a payment of $6,329. As of December 31, 20202021 totaled $2,370, $17,114 and 2019, respectively, there were 183,078 and 333,330 vested awards outstanding both with a grant date fair value of $4.89.
Profits interest compensation of $10,103, $10,844 and $14,325 for all plans, was recognized for the years ended December 31, 2020, 2019 and 2018$19,504, respectively. The expense is primarily included in selling, general and administrative expense andwith a nominal amount in research and development expense on the consolidated statementstatements of operations and comprehensive (loss) income (loss) based upon the classification of the employee. As ofThere were no income tax benefits related to equity-based compensation expense for the years ended December 31, 2020, there was approximately $9,741 of unrecognized2023 and 2021. Income tax benefits totaled $2,951 related to compensation expense to be recognized over a weighted-average period of 1.3 years.
A summary of the award activity of the Phantom Plan for the year ended December 31, 20202022.
Restricted Stock Units
During the years ended December 31, 2023 and 2022, the Company granted time-based RSUs which vest at various dates through December 4, 2027. RSU compensation expense is recognized over the vesting period, which is typically between 1 and 4 years. Unamortized compensation expense related to the RSUs totaled $3,859 at December 31, 2023, and is expected to be recognized over a weighted average period of approximately 1.92 years. A summary of the RSU award activity for the years ended December 31, 2023 and 2022 are as follows (number of awardsunits in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2012 Phantom Units | | 2015 Phantom Units |
| (awards in thousands) | Number of awards | | Weighted average grant-date fair value | | Number of awards | | Weighted average grant-date fair value |
| Outstanding at December 31, 2019 | 658 | | | $ | 5.72 | | | 1,139 | | | $ | 10.24 | |
| Granted | — | | | $ | — | | | 553 | | | $ | 10.29 | |
| Converted to cash | — | | | $ | — | | | (146) | | | $ | 6.45 | |
| Forfeited | — | | | $ | — | | | (124) | | | $ | 12.98 | |
| Outstanding at December 31, 2020 | 658 | | | $ | 5.72 | | | 1,422 | | | $ | 10.41 | |
| Awards vested at December 31, 2020 | 658 | | | $ | 5.72 | | | 495 | | | $ | 8.15 | |
| | | | | | | | | | | |
| Number of units | | Weighted-average grant-
date fair value per unit |
| Unvested at December 31, 2021 | 1,024 | | | $ | 14.41 | |
| Granted | 1,248 | | | 10.73 | |
| Vested | (756) | | | 14.74 | |
| Forfeited or canceled | (327) | | | 8.45 | |
| Unvested at December 31, 2022 | 1,189 | | | 11.96 | |
| Granted | 2,067 | | | 2.34 | |
| Vested | (641) | | | 8.74 | |
| Forfeited or canceled | (549) | | | 8.53 | |
| Unvested at December 31, 2023 | 2,066 | | | $ | 4.51 | |
There were no 2012 Phantom Unit awardsStock Options
During the years ended December 31, 2023 and 2022, the Company granted time-based stock options which vest over 1 to 4 years following the date of grant and expire within 10 years. The fair value of time-based stock options is determined using the Black-Scholes valuation model, with such value recognized as expense over the service period, which is typically 1 to 4 years, net of actual forfeitures. A summary of the Company’s assumptions used in 2019. determining the fair value of the stock options granted during the years ended December 31, 2023 and 2022 are shown in the following table:
| | | | | | | | | | | |
| 2023 | | 2022 |
| Risk-free interest rate | 3.5% - 4.7% | | 1.8% - 4.3% |
| Expected dividend yield | — | % | | — | % |
| Expected stock price volatility | 35.2% - 36.4% | | 33.2% - 35.2% |
| Expected life of stock options (years) | 5.50 - 6.25 | | 6.25 |
The weighted averageweighted-average grant date fair value per Other Phantom Unit awardsof options granted induring the year ended December 31, 20192023 was $15.31.$0.53 per share. The expected term of the options granted is estimated using the simplified method. Expected volatility is based on the historical volatility of the Company’s peers’ common stock. The risk-free interest rate is determined based upon a constant U.S. Treasury security rate with a contractual life that approximates the expected term of the option. Unamortized compensation expense related to the options totaled $2,206 at December 31, 2023, and is expected to be recognized over a weighted average period of approximately 2.26 years.
| | | | | | | | | | | | | | | | | | | | | | | |
| 2012 Phantom Units | | 2015 Phantom Units |
| (awards in thousands) | Number of awards | | Weighted average grant-date fair value | | Number of awards | | Weighted average grant-date fair value |
| Nonvested at December 31, 2019 | 2 | | | $ | 10.01 | | | 667 | | | $ | 12.71 | |
| Vested during 2020 | 2 | | | $ | 10.01 | | | 147 | | | $ | 10.81 | |
| Nonvested at December 31, 2020 | — | | | $ | — | | | 927 | | | $ | 11.62 | |
The total fair valueA summary of 2012 Phantom Unit awards vested instock option activity is as follows for the yearyears ended December 31, 2020 was nominal. 2023 and 2022 (number of options in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of options | | Weighted-average exercise price | | Weighted average remaining contractual term (in years) | | Aggregate intrinsic value |
| Outstanding at December 31, 2021 | 8,364 | | | $ | 11.16 | | | | | |
| Granted | 2,606 | | | 12.19 | | | | | |
| Exercised | (680) | | | 6.28 | | | | | |
| Forfeited or canceled | (1,380) | | | 12.38 | | | | | |
| Outstanding at December 31, 2022 | 8,910 | | | 11.65 | | | 7.74 | | $ | 3 | |
| Granted | 1,226 | | | 1.28 | | | | | |
| Exercised | (67) | | | 2.68 | | | | | |
| Forfeited or canceled | (5,722) | | | 11.80 | | | | | |
| Outstanding at December 31, 2023 | 4,347 | | | 8.68 | | | 7.31 | | $ | 4,383 | |
| Exercisable and vested at December 31, 2023 | 1,958 | | | $ | 10.25 | | | 4.45 | | $ | 144 | |
The total fairaggregate intrinsic value is calculated as the difference between the exercise price of Other Phantom Unit awards vestedthe underlying options and the market price of the Company’s Class A common stock for options that had exercise prices lower than $5.27 per share, the closing price of the Company’s Class A common stock on December 29, 2023.
Employee Stock Purchase Plan
The Company operates a non-qualified Employee Stock Purchase Plan (“ESPP”), which provides for the issuance of shares of the Company’s Class A common stock to eligible employees of the Company that elect to participate in the yearplan and purchase shares of Class A common stock through payroll deductions at a discounted price. As of December 31, 2023, the aggregate number of shares reserved for issuance under the ESPP was 886,930. A total of 516,976, 279,000 and 94,795 shares were issued and $352, $471 and $340 of expense was recognized during the years ended December 31, 2020 was $4,703.2023, 2022 and 2021, respectively.
Defined contribution plans
The Company has various defined contribution plans or plans that share profit which are offered in Canada, Germany, the Netherlands, United Kingdom and the United Kingdom.Israel. These plans are required by local laws or regulations in some cases. Contributions are primarily discretionary, except in some countries where contributions are contractually required. These plans cover substantially all eligible employees in the countries where the plans are offered either voluntarily or statutorily.
In the U.S.,United States, the Company provides a 401(k) defined contribution plan (U.S. Plan)(“U.S. Plan”) that covers substantially all U.S. employees that meet minimum age requirements. The Company matches 50%100% of the employees’ contribution up to 6% of the employees’ wages. The Company also contributes 4.5%4% of the employees’ wages and 50% on the next 2%. The U.S. Plan also provides for an additional 1% to 3% at the U.S. Plan. The 4.5% Company’s discretion.
Company contribution was suspended in May 2020 due to the COVID-19 crisiscontributions totaled $5,836, $6,407, and reinstated in late December 2020.
For$4,477 for all global plans during the years ended December 31, 2020, 20192023, 2022 and 2018, Company contributions totaled $3,379, $5,401 and $5,462 respectively, for all global plans.2021, respectively. The expense is included in cost of sales, selling, general and administrative expense and research and development expense on the consolidated statement of operations and comprehensive (loss) income (loss) based upon the classification of the employee.
8. Stockholders’ equity
Initial Public Offering
On February 16, 2021, the Company closed an IPO of 9,200,000 shares of Class A common stock at a public offering price of $13.00 per share, which includes 1,200,000 shares issued pursuant to the underwriters' over-allotment option. The Company received $111,228 in proceeds, net of underwriting discounts and commissions of $8,372, which was used to purchase newly-issued membership interests from BV LLC at a price per interest equal to the IPO price of $13.00. The Company also incurred offering expenses totaling $4,778 in addition to the underwriting discounts and commissions. Offering expenses of $1,327 were paid in 2020 and $3,451 were paid in 2021. The Company is the sole managing member of, has a majority economic interest in, has the sole voting interest in, and controls the management of BV LLC. As a result, the Company consolidates the financial results of BV LLC and reports a non-controlling interest for the interest not held by the Company.
IPO Transactions
In connection with the IPO, the Company completed the following transactions (“Transactions”).
•Amended and restated the limited liability company agreement of BV LLC (“BV LLC Agreement”), to, among other things, (i) provide for a new single class of common membership interests in BV LLC (“LLC Interests”); (ii) exchange all of the existing membership interests in BV LLC (“Original BV LLC Owners”) for new LLC Interests; and (iii) appoint Bioventus Inc. as the sole managing member of BV LLC.
•Amended and restated the Bioventus Inc. certificate of incorporation to, among other things, (i) provide for an increase in the authorized shares of Class A common stock; (ii) provide for Class B common stock with voting rights but no economic interest, which shares were issued to the Original BV LLC Owners on a one-for-one basis with the number of LLC Interests they owned; and (iii) provide for undesignated preferred stock.
•Acquired, by merger, ten entities that were Original BV LLC Owners (“Former LLC Owners”), for which the Company issued 31,838,589 shares of Class A common stock as merger consideration (“IPO Mergers”). The only assets held by the Former LLC Owners were 31,838,589 LLC Interests and a corresponding number of shares of Class B common stock. Upon consummation of the IPO Mergers, the 31,838,589 shares of Class B common stock were canceled, and the Company recognized the 31,838,589 LLC Interests at carrying value, as the IPO Mergers are considered to be a recapitalization transaction. The Company issued 15,786,737 shares of Class B common stock to the Original BV LLC Owners.
The financial statements for periods prior to the IPO and Transactions have been adjusted to combine the previously separate entities for presentation purposes. Prior to the Transactions, Bioventus Inc. had no operations.
Amendment and restatement of certificate of incorporation
On February 16, 2021, the Company amended and restated its certificate of incorporation to, among other things, provide for: (i) the authorization of 250,000,000 shares of Class A common stock with a par value of $0.001 per share; (ii) the authorization of 50,000,000 shares of Class B common stock with a par value of $0.001 per share; (iii) the authorization of 10,000,000 shares of undesignated preferred stock that may be issued from time to time by the BOD in one or more series; and (iv) the establishment of a classified BOD, divided into three classes, each of whose members will serve for staggered three-year terms.
Holders of Class A and Class B common stock are entitled to one vote per share and, except as otherwise required, will vote together as a single class on all matters on which stockholders generally are entitled to vote. Holders of Class B common stock are not entitled to receive dividends and will not be entitled to receive any distributions upon the liquidation, dissolution or winding up of the Company. Shares of Class B common stock may only be issued to the extent necessary to maintain the one-to-one ratio between the number of LLC Interests and the number of shares of Class B common stock held by the Continuing LLC Owner. Shares of Class B common stock are transferable only together with an equal number of LLC Interests. Shares of Class B common stock will be canceled on a one-for-one basis upon the redemption or exchange of any outstanding LLC Interests.
BV LLC recapitalization
On February 16, 2021, the Company amended and restated the BV LLC Agreement to, among other things, (i) provide for new LLC Interests; (ii) exchange all of the then existing membership interests of the Original BV LLC Owners for new LLC Interests; and (iii) appoint Bioventus Inc. as the sole managing member of BV LLC.
The BV LLC Agreement provides that holders of LLC Interests may, from time to time, require the Company to redeem all or a portion of their LLC Interests for newly-issued shares of Class A common stock on a one-for-one basis. The Company may elect to settle any such redemption in shares of Class A common stock or in cash.
The amendment also requires that the Company, at all times, maintain (i) a one-to-one ratio between the number of outstanding shares of Class A common stock and the number of LLC Interests owned by the Company and (ii) a one-to-one ratio between the number of shares of Class B common stock owned by the Continuing LLC Owner and the number of LLC Interests owned by the Continuing LLC Owner.
Noncontrolling interest
In connection with any redemption of LLC Interests by the Continuing LLC Owner, the Company will receive a corresponding number of LLC Interests, increasing its ownership interest in BV LLC. Future redemptions of LLC Interests will result in a change in ownership and reduce the amount recorded as noncontrolling interest and increase additional paid-in capital. There were no redemptions during the years ended December 31, 2023 and 2022. The following table summarizes the ownership interest in BV LLC as of December 31 (number of units in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2023 | | 2022 |
| LLC Interests | | Ownership % | | LLC Interests | | Ownership % |
| Number of LLC Interests owned | | | | | | | |
| Bioventus Inc. | 63,267 | | | 80.0 | % | | 62,063 | | | 79.7 | % |
| Continuing LLC Owner | 15,787 | | | 20.0 | % | | 15,787 | | | 20.3 | % |
| Total | 79,054 | | | 100.0 | % | | 77,850 | | | 100.0 | % |
9. Earnings per share
The following table sets forth the computation of basic and diluted loss per share of Class A common stock for the periods presented (amounts in thousands, except share and per share data):
| | | | | | | | | | | | | | | | | |
| December 31, 2023 | | December 31, 2022 | | February 16, 2021 through the Year Ended December 31, 2021 |
| Numerator: | | | | | |
| Net loss from continuing operations | $ | (121,196) | | | $ | (144,651) | | | $ | (14,523) | |
Net loss attributable to noncontrolling interests —
continuing operations | 24,458 | | | 40,732 | | | 9,789 | |
Net loss attributable to Bioventus Inc. Class A
common stockholders — continuing operations | $ | (96,738) | | | $ | (103,919) | | | $ | (4,734) | |
| | | | | |
| Numerator: | | | | | |
| Net loss from discontinued operations | $ | (74,429) | | | $ | (68,740) | | | $ | (1,868) | |
Net loss attributable to noncontrolling interests —
discontinued operations | 14,937 | | | 13,955 | | | — | |
Net loss attributable to Bioventus Inc. Class A
common stockholders — discontinued operations | $ | (59,492) | | | $ | (54,785) | | | $ | (1,868) | |
| | | | | |
| Denominator: | | | | | |
Weighted-average shares of Class A common stock
outstanding - basic and diluted | 62,647,554 | | | 61,389,107 | | | 45,472,483 | |
| | | | | |
Net loss per share of Class A common stock, from
continuing operations, basic and diluted | $ | (1.54) | | | $ | (1.70) | | | $ | (0.11) | |
Net loss per share of Class A common stock, from
discontinued operations, basic and diluted | (0.95) | | | (0.89) | | | (0.04) | |
Net loss per share of Class A common stock,
basic and diluted | $ | (2.49) | | | $ | (2.59) | | | $ | (0.15) | |
Shares of Class B common stock do not share in the losses of the Company and are therefore not participating securities. As such, separate presentation of basic and diluted losses per share of Class B common stock under the two-class method has not been presented.
The following number of weighted-average potentially dilutive shares as of December 31, 2023 and 2022 were excluded from the calculation of diluted loss per share because the effect of including such potentially dilutive shares would have been antidilutive upon conversion:
| | | | | | | | | | | | | | | | | |
| Year Ended
December 31, 2023 | | Year Ended
December 31, 2022 | | February 16, 2021 through the Year Ended December 31, 2021 |
LLC Interests held by Continuing LLC Owner(a) | 15,786,737 | | | 15,786,737 | | | 15,786,737 | |
| Stock options | 5,860,516 | | | 7,679,780 | | | 5,373,442 | |
| RSUs | 595,030 | | | 710,807 | | | 966,673 | |
| Unvested shares of Class A common stock | — | | | — | | | 30,056 | |
| Total | 22,242,283 | | | 24,177,324 | | | 22,156,908 | |
(a)Class A Shares reserved for future issuance upon redemption or exchange of LLC Interests by the Continuing LLC Owner.
10. Restructuring costs
Restructuring costs are not allocated to the Company’s reportable segments as they are not part of the segment performance measures regularly reviewed by management. These charges are included in restructuring costs in the consolidated statements of operations and comprehensive (loss) income. Liabilities associated from restructuring costs are recorded in accrued liabilities on the consolidated balance sheets.
The Company announced a restructuring plan in December 2022 (“2022 Restructuring Plan”) that was intended to align the Company’s organizational and management cost structure to improve profitability and cash flow. The 2022 Restructuring Plan primarily consisted of employee severance and additional expenses for third-party and other related costs. Pre-tax charges recognized during the year ended December 31, 2023 and 2022 totaled $793 and $4,581, respectively. The 2022 Restructuring Plan is complete.
The Company adopted restructuring plans for businesses acquired to reduce headcount, reorganize management structure and consolidate certain facilities during the second half of 2021 (“2021 Acquisition Restructuring Plan”) and during the first quarter of 2022 (“2022 Acquisition Restructuring Plan”). The Company planned total pre-tax charges for the 2021 Acquisition Restructuring Plan and the 2022 Acquisition Restructuring Plan were $3,500 and $2,300, respectively. There was nominal activity related to the 2021 Acquisition Restructuring Plan during the year ended December 31, 2023, and $719 and $2,487 was recognized during the years ended December 31, 2022 and 2021, respectively. The 2021 Acquisition Restructuring Plan is complete. Costs attributable to the 2022 Acquisition Restructuring Plan totaled $84 and $1,479 during the years ended December 31, 2023 and 2022, respectively.
The Company’s restructuring charges and payments for plans related to businesses acquired comprised of the following:
| | | | | | | | | | | | | | | | | |
| Employee severance and temporary labor costs | | Other charges | | Total |
| Balance at December 31, 2021 | $ | 1,400 | | | $ | 136 | | | $ | 1,536 | |
| Expenses incurred | 5,172 | | | 1,607 | | | 6,779 | |
| Payments made | (2,812) | | | (1,743) | | | (4,555) | |
| Balance at December 31, 2022 | 3,760 | | | — | | | 3,760 | |
| Expenses incurred | 648 | | | 192 | | | 840 | |
| Payments made | (3,092) | | | (192) | | | (3,284) | |
| Balance at December 31, 2023 | $ | 1,316 | | | $ | — | | | $ | 1,316 | |
11. Income taxes
As a result of the Transactions, Bioventus Inc. became the sole managing member of BV LLC, which is treated as a partnership for income tax purposes. As a partnership, BV LLC is not subject to United States federal and certain state and local income taxes. Any taxable income or loss generated by BV LLC is passed through to and included in the taxable income or loss of its members, including the Company, following the Transactions, on a pro rata basis. Prior to the Transactions, income from other domestic subsidiaries included BV LLC and thereafter is included in income from domestic taxable subsidiaries. The components of net(loss) income from continuing operations before income taxes for the years ended December 31 are as follows:
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Taxable subsidiaries: | | | | | |
| Domestic | $ | 306 | | | $ | 2,679 | | | $ | 2,925 | |
| Foreign | 387 | | | 2,967 | | | (1,393) | |
| 693 | | | 5,646 | | | 1,532 | |
| Other domestic subsidiaries | 15,221 | | | 4,043 | | | 4,575 | |
| Income from continuing operations before income taxes | $ | 15,914 | | | $ | 9,689 | | | $ | 6,107 | |
| | | | | | | | | | | | | | | | | |
| 2023 | | 2022 | | 2021 |
| United States | $ | (125,676) | | | $ | (187,571) | | | $ | 9,511 | |
| International | 4,565 | | | (1,454) | | | (23) | |
| (Loss) income before income taxes - continuing operations | $ | (121,111) | | | $ | (189,025) | | | $ | 9,488 | |
Table ofcontentsThe provision for income taxes on operations consists of the following for the years ended December 31:
| | | | | | | | | | | | | | | | | |
| 2023 | | 2022 | | 2021 |
| Current: | | | | | |
| United States federal | $ | (734) | | | $ | 2,092 | | | $ | 5,675 | |
| United States state and local | 2,085 | | | (96) | | | 1,750 | |
| International | 1,111 | | | 296 | | | 367 | |
| Total current | 2,462 | | | 2,292 | | | 7,792 | |
| | | | | |
| Deferred: | | | | | |
| United States federal | (2,881) | | | (38,678) | | | (9,015) | |
| United States state and local | (1,951) | | | (7,897) | | | (533) | |
| International | 2,455 | | | (91) | | | (210) | |
| Total deferred | (2,377) | | | (46,666) | | | (9,758) | |
| Total income tax expense (benefit) - continuing operations | $ | 85 | | | $ | (44,374) | | | $ | (1,966) | |
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Federal income taxes: | | | | | |
| Current | $ | 782 | | | $ | 932 | | | $ | 891 | |
| Deferred | (508) | | | (345) | | | (294) | |
| Foreign income taxes: | | | | | |
| Current | 707 | | | 815 | | | 472 | |
| Deferred | — | | | — | | | 180 | |
| State income taxes: | | | | | |
| Current | 214 | | | 177 | | | 380 | |
| Deferred | (3) | | | (3) | | | (1) | |
| Change in tax rates - deferred | — | | | — | | | 36 | |
| Income tax expense | $ | 1,192 | | | $ | 1,576 | | | $ | 1,664 | |
The differences between the effective income tax rate and the federal statutory income tax rates for the years ended December 31 by taxable and other subsidiaries are as follows:
| | 2020 | | 2019 | | 2018 |
| 2023 | | | 2023 | | 2022 | | 2021 |
| U.S. statutory federal corporate income tax rate | U.S. statutory federal corporate income tax rate | 21.0 | % | | 21.0 | % | | 21.0 | % | U.S. statutory federal corporate income tax rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| Noncontrolling interest | |
| LLC flow-through structure | LLC flow-through structure | (20.1) | | | (8.8) | | | (15.7) | |
| Non-deductible expenses | |
| State and local income taxes, net of federal benefit | State and local income taxes, net of federal benefit | 1.5 | | | 2.4 | | | 7.3 | |
| Change in valuation allowance | |
| Research and other tax credits | |
| Organizational Transactions | |
| Uncertain tax positions | |
| Foreign rate differential | Foreign rate differential | 1.2 | | | 1.7 | | | 11.5 | |
| Provision to return adjustment | 3.9 | | | — | | | 3.1 | |
| GAAP impairment | |
| Stock compensation | |
| Other | |
| Effective income tax rate | Effective income tax rate | 7.5 | % | | 16.3 | % | | 27.2 | % | Effective income tax rate | (0.1 | %) | | 23.5 | % | | (25.8 | %) |
The Company’s effective tax rate differs from statutory rates primarily due to Bioventus LLC’s pass-through structure for U.S. income tax purposes while being treated as taxable in certain states and various foreign jurisdictions as well as for certain subsidiaries. In addition, certain states assess income taxes on pass-through structures.
Deferred tax assets and liabilities are determined based on the difference between financial statement and tax bases using enacted tax rates in effect for the year in which the differences are expected to reverse. The components of the deferred taxes were as follows:
| | 2020 | | 2019 |
| 2023 | | | 2023 | | 2022 |
| Deferred tax assets: | Deferred tax assets: | | | |
| Net operating losses | $ | 3,874 | | | $ | 3,530 | |
| Tax credit carryforwards and other | 696 | | | 390 | |
| Gross deferred tax assets | 4,570 | | | 3,920 | |
| Capital loss carryforward | |
| Capital loss carryforward | |
| Capital loss carryforward | |
| Interest | |
| Net operating losses and tax credit carryforwards | |
| Tax credits | |
| Investment in Bioventus LLC | |
| Transaction costs | |
| Stock-based compensation | |
| Research & Development | |
| Fixed assets | |
| Accrued liabilities | |
| Other | |
| Gross deferred tax asset | |
| Valuation allowance | Valuation allowance | (2,993) | | | (2,423) | |
| Total deferred tax assets | Total deferred tax assets | 1,577 | | | 1,497 | |
| Deferred tax liability: | Deferred tax liability: | |
| Acquired intangible | 4,939 | | | 5,371 | |
| Investment in Bioventus LLC | |
| Investment in Bioventus LLC | |
| Investment in Bioventus LLC | |
| Intangibles | |
| Gross deferred tax liability | |
| Net deferred tax liability | Net deferred tax liability | $ | 3,362 | | | $ | 3,874 | |
The valuation allowance is primarily attributable to net operating losses (“NOLs”). The Company considered many factors when assessing the likelihood of future realization of these deferred tax assets, including expectations of future taxable income or loss, the carryforward periods available to the Company for tax reporting purposes, and other relevant factors. The net change in the valuation allowance was $43,471. The valuation allowance at December 31, 2023 is primarily against NOLs, interest carryover and capital loss carryforward. The valuation allowance at December 31, 2022 principally relates to recognizing a full valuation allowance against foreign NOLs.
Beginning in 2022, the Tax Cuts and Jobs Act of 2017 eliminated the option to deduct research and development expenditures immediately in the year incurred and requires companies to amortize such expenditures over five or fifteen years for tax purposes, depending on whether the activities were incurred in the United States or outside of the United States. This change resulted in an increase to gross deferred tax assets and cash tax liabilities.
As of December 31, 2023, the Company had approximately $42,422 in U.S. federal NOL carryforwards at its corporate subsidiaries and $1,213 in federal tax credits. Certain US federal NOL carryforwards begin to expire in 2031, while others were generated after the enactment of the Tax Cuts and Jobs Act (the “Act”) and as such do not expire, but can only be utilized to offset up to 80% of taxable income in any given year. The federal tax credits start to expire at various dates beginning in 2026. As of December 31, 2023, the Company had approximately $27,695 in state NOL carryforwards and $354 in state tax credits. If not utilized, some state NOL carryforwards will expire at various dates beginning in 2025.
The Company assessesevaluated its tax positions and had unrecognized tax benefits of $4,725 and $5,883 as of December 31, 2023 and 2022, respectively. The Company had $2,047 and $2,297 accrued for payment of interest and penalties as of December 31, 2023 and 2022, respectively. If the need for a$4,725 of unrecognized tax benefit is recognized, it would not impact the effective tax rate due to the valuation allowance against our deferred tax assets. A valuation allowance of $2,822, has been applied to all of Harbor’son the Company's net U.S. deferred tax assets. The Company also hasdoes expect a valuation allowancematerial decrease of $171 representingapproximately $1,446 in the entire balance of deferred tax assets relating to our international operations. These valuation allowances were recorded as the Company believes it is more-likely-than-not that it will receive future benefit. Attwelve months following December 31, 2020, the Company had federal and state net operating loss carryforwards related to Harbor of $25,537 expiring at various dates from 2021 through 2037 and approximately $2,141 with no expiration date.
The Company evaluates2023 in its tax positions in accordance with the recognition and measurement provisions of ASC 740-10 (“FIN 48”) and in accordance with that guidance, has determined that a reserve for any significant uncertain tax positions due to various statute expirations during 2024. The Company files U.S. federal income tax returns as well as income tax returns in many United States and foreign jurisdictions. In general, the tax years 2020 - 2023 remain open to examination by the major jurisdictions in which the Company is not needed as of December 31, 2020 and 2019.subject to tax.
Under current tax law, the utilization of tax attributes will be restricted if an ownership change, as defined, were to occur. Section 382A reconciliation of the U.S. Internal Revenue Code of 1986, as amended, or the Internal Revenue Code, provides, in general, that if an “ownership change” occurs with respect to a corporation with net operatinggross unrecognized tax benefits (excluding interest and other loss carryforwards, such carryforwards will be available to offset taxable income in each taxable year after the ownership change only up to the “Section 382 Limitation”penalties) for each year. The Company’s ability to use its loss carryforwards will be limited in the event of an ownership change.
During the year ended December 31, 2018, Dutch income taxes were imposed on a negotiated percentage of sales. The Company has an agreement with the Dutch taxing authorities where the Company’s Netherlands subsidiary will incur but not have to pay income taxes in years when the subsidiary is operating at a loss.2023 follows:
Minimal tax related interest and penalties were incurred for the years ended December 31, 2020 and 2019. The Company is subject to audit by various taxing jurisdictions for the years 2015 through 2020. | | | | | |
| Beginning of the period | $ | 5,883 | |
| Additions for current year tax positions | 142 | |
| Expiration of statutes | (1,300) | |
| End of the period | $ | 4,725 | |
11. Related-party transactionsTax Receivable Agreement
The Company made cash tax distributions of $19,886, $9,137 and $7,846expects to its membersobtain an increase in an amount equal to approximately 40%the share of the members’ estimated taxable incometax basis of the assets of BV LLC when LLC Interests are redeemed or exchanged by the Continuing LLC Owner and other qualifying transactions. This increase in tax basis may have the effect of reducing the amounts that the Company would otherwise pay in the future to various tax authorities. The increase in tax basis may also decrease gains (or increase losses) on future dispositions of certain capital assets to the extent tax basis is allocated to those capital assets.
On February 16, 2021, the Company entered into a tax receivable agreement (“TRA”) with the Continuing LLC Owner that provides for the years endedpayment by the Company to the Continuing LLC Owner of 85% of the amount of tax benefits, if any, that the Company actually realizes as a result of (i) increases in the tax basis of assets of BV LLC resulting from any redemptions or exchanges of LLC Interests or any prior sales of interests in BV LLC; and (ii) certain other tax benefits related to the Company making payments under the TRA.
The Company will maintain a full valuation allowance against deferred tax assets related to the tax attributes generated as a result of redemptions of LLC Interests or exchanges described above until it is determined that the benefits are more-likely-than-not to be realized. As of December 31, 2020, 20192023, the Continuing LLC Owner had not exchanged LLC Interests for shares of Class A common stock and 2018, respectively. At December 31, 2020 and December 31, 2019, there were tax distributions payable to tax authorities ontherefore the members’ behalf totaling $541 and $473, respectively, and nominal tax distributions payable toCompany had not recorded any liabilities under the members.TRA.
12. Commitments and contingencies
Leases
The Company determines if an arrangement is a lease at inception. The Company leases its office facilities as well as other property, vehicles and equipment under operating leases. The Company also leases certain office equipment under nominal finance leases. Lease assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the obligation to make lease payments arising from the lease. Lease assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company’s incremental borrowing rate is used as a discount rate, based on the information available at the commencement date, in determining the present value of lease payments. Lease assets also include the impact of any prepayments made and are reduced by impact of any lease incentives.
The Company does not recognize lease liabilities or lease assets on the balance sheet for short-term (leases with a lease term of twelve months or less as of the commencement date). Rather, any short-term lease payments are recognized as an expense on a straight-line basis over the lease term. The current period short-term lease expense reasonably reflects short-term lease commitments.
For all classifications of leases, the Company combines lease and nonlease components in order to record the combination as a single lease component. Variable lease payments are excluded from the lease liability and recognized in the period in which the obligation is incurred. Additionally, lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise the option.
The Company leases its office facilities as well as other property, vehicles and equipment under operating leases. The Company also leases certain office equipment under nominal finance leases. The remaining lease terms range from 3 months1 month to 89.33 years.
The components of lease cost were as follows:follows for the years ended December 31:
| | 2020 | | 2019 |
| | | | | 2023 | |
| | | | | 2023 | |
| | | | | 2023 | | | 2022 | | 2021 |
| Operating lease cost | Operating lease cost | $ | 2,610 | | | $ | 2,529 | |
| Short-term lease cost* | 388 | | | 358 | |
Short-term lease cost(a) | |
| Financing lease cost: | |
| Amortization of finance lease assets | |
| Amortization of finance lease assets | |
| Amortization of finance lease assets | |
| Interest on lease liabilities | |
| Total lease cost | Total lease cost | $ | 2,998 | | | $ | 2,887 | |
*(a)Includes variable lease cost and sublease income, which are immaterial.
Supplemental cash flow information and non-cash activity related to operating leases were as follow:follows for the years ended December 31:
| | | | | | | | | | | |
| 2020 | | 2019 |
| Cash paid for amounts included in the measurement of operating lease liabilities: | $ | 2,567 | | | $ | 2,343 | |
| Right-of-use assets obtained in exchange for operating lease obligations: | $ | 1,497 | | | $ | 5,016 | |
| | | | | | | | | | | | | | | | | |
| 2023 | | 2022 | | 2021 |
| Cash paid for amounts included in measurement of lease liabilities: | | | | | |
| Operating cash flows from operating leases | $ | 4,516 | | | $ | 4,374 | | | $ | 3,616 | |
| Operating cash flows from financing leases | $ | 793 | | | $ | 5 | | | $ | 5 | |
| Financing cash flows from finance leases | $ | 506 | | | $ | 53 | | | $ | 37 | |
| | | | | |
| Right-of-use assets obtained in exchange for lease obligations: | | | | | |
| Operating lease obligations | $ | 344 | | | $ | 4,792 | | | $ | 4,665 | |
| Finance lease obligations | $ | 15,567 | | | $ | — | | | $ | 114 | |
CurrentSupplemental balance sheet and noncurrentother information related to operating leases were as follows for the years ended December 31:
| | | | | | | | | | | |
| 2023 | | 2022 |
| Operating lease assets | $ | 13,353 | | $ | 16,690 |
| | | |
| Operating lease liabilities- current | $ | 4,057 | | $ | 3,552 |
| Operating lease liabilities- noncurrent | 10,573 | | 14,355 |
| Total operating lease liabilities | $ | 14,630 | | $ | 17,907 |
| | | |
| Financing leases | | | |
| Property, plant and equipment - net | $ | 14,279 | | $ | 128 |
| | | |
| Finance lease liabilities - current | $ | 759 | | $ | 55 |
| Finance lease liabilities - noncurrent | 10,386 | | 76 |
| Total financing lease liabilities | $ | 11,145 | | $ | 131 |
| | | |
| Weighted average remaining lease term (years): | | | |
| Operating leases | 3.8 | | 4.8 |
| Finance leases | 9.3 | | 2.4 |
| Weighted average discount rate: | | | |
| Operating leases | 4.7 | % | | 4.8 | % |
| Finance leases | 8.1 | % | | 3.3 | % |
Future maturities of operating and finance lease liabilities are included in other current liabilities and other long-term liabilities, respectively, on the consolidated balance sheet. Other balance sheet information related to leases are as follows:
| | | | | | | | | | | |
| 2020 | | 2019 |
| Operating lease assets | $ | 14,961 | | $ | 15,267 |
| Operating lease liabilities—current | $ | 1,960 | | $ | 1,814 |
| Operating lease liabilities—noncurrent | 14,108 | | 14,513 |
| Total operating lease liabilities | $ | 16,068 | | $ | 16,327 |
| Weighted average remaining operating lease term in years | 7.2 | | 8.0 |
| Weighted average discount rate for operating leases | 5.0 | % | | 5.0 | % |
| | | | | | | | | | | |
| Operating leases | | Finance Leases |
| 2024 | $ | 4,565 | | | $ | 1,623 | |
| 2025 | 4,078 | | | 1,617 | |
| 2026 | 3,245 | | | 1,630 | |
| 2027 | 2,619 | | | 1,662 | |
| 2028 | 1,497 | | | 1,696 | |
| Thereafter | — | | | 7,753 | |
| Total undiscounted cash flows | 16,004 | | | 15,981 | |
| Less imputed interest | (1,374) | | | (4,836) | |
| Present value of future lease payments | $ | 14,630 | | | $ | 11,145 | |
MaturitiesIn the normal course of lease liabilities as of December 31, 2020 were as follows:
| | | | | |
| Operating leases |
| 2021 | $ | 2,714 | |
| 2022 | 2,645 | |
| 2023 | 2,460 | |
| 2024 | 2,476 | |
| 2025 | 2,569 | |
| Thereafter | 6,281 | |
| Total future lease payments | 19,145 | |
| Less imputed interest | (3,077) | |
| Present value of future lease payments | $ | 16,068 | |
OIG’s Provider Self-Disclosure
business, the Company periodically becomes involved in various claims and lawsuits, and governmental proceedings and investigations that are incidental to its business. The Company identified non-compliance with certain U.S. federal statutesaccrues a liability when a loss is considered probable and requirements governing the Medicare program in 2018 related to improper completion of Certificate for Medical Necessity (CMN) formsamount can be reasonably estimated. When a material loss contingency is reasonably possible but not probable, the Company does not record a liability, but instead discloses the nature and in November 2018 made a voluntary self-disclosure to the Office of Inspector Generalamount of the U.S. Departmentclaim, and an estimate of Healththe possible loss or range of loss, if such an estimate can be made. Legal fees are expensed as incurred. With respect to governmental proceedings and Human Services (OIG) pursuantinvestigations, like other companies in the industry, the Company is subject to the OIG’s Provider Self-Disclosure Protocol related to this matter. After settlement discussions with the Office ofextensive regulation by national, state and local governmental agencies in the United States Attorneyand in other jurisdictions in which the Company and its affiliates operate. As a result, interaction with governmental agencies is ongoing. The Company’s standard practice is to cooperate with regulators and investigators in responding to inquiries.
The Company is presently unable to predict the duration, scope, or result of these matters. As such, the Company is presently unable to develop a reasonable estimate of a possible loss or range of losses, if any, related to these matters. While the Company intends to defend these matters vigorously, the outcome of such litigation or any other litigation is necessarily uncertain, is not within the Company’s complete control and might not be known for extended periods of time. In the opinion of management, the outcome of any existing claims and legal or regulatory proceedings, other than the specific matters described below, if decided adversely, is not expected to have a material adverse effect on the Company's business, financial condition, results of operations, or cash flows.
Bioventus stockholder litigation
On January 12, 2023, the Company and certain of its current and former directors and officers were named as defendants in a putative class action lawsuit filed in the Middle District of North Carolina, (USAO)Ciarciello v. Bioventus, Inc., No. 1:23– CV – 00032-CCE-JEP (M.D.N.C. 2023). The complaint asserts violations of Sections 10(b) and OIG, on January20(a) of the Exchange Act and of Sections 11 and 15 2021,of the Securities Act and generally alleges that the Company reached a settlement agreementfailed to disclose certain information regarding rebate practices, its business and financial prospects, and the sufficiency of internal controls regarding financial reporting. The complaint seeks damages in an unspecified amount. On April 12, 2023, the Court appointed Wayne County Employees’ Retirement System as lead plaintiff. The plaintiff’s amended consolidated complaint was filed with the USAOCourt on June 12, 2023. On July 17, 2023, the defendants filed a motion to dismiss the complaint raising a number of legal and factual deficiencies with the OIG with respectamended consolidated complaint. In response to the submission of Medicare claims that did not meet CMS coverage requirements and fordefendants’ motion to dismiss, the lead plaintiff filed a second amended complaint on July 31, 2023. The defendants moved to dismiss the second amended complaint on August 21, 2023, which the Company’s sales representatives completed Section BCourt granted in part and denied in part on November 6, 2023. The Court dismissed the plaintiff’s Securities Act claims, but allowed the plaintiff’s Exchange Act claims to proceed into discovery.
On October 4, 2023, certain of the CMN form. Company’s current and former directors and officers were named as defendants in a derivative shareholder lawsuit (in which the Company is a nominal defendant) filed in the United States District Court for the District of Delaware, Grogan, on behalf of Bioventus Inc., v. Reali, et.al., No. 1:23-CV-01099-RGA (D. Del. 2023). The complaint asserts violations of Section 14(a) of the Exchange Act, breaches of fiduciary duties and related state law claims, and a claim for contribution, and generally alleges the same purported misconduct as alleged in the Ciarciello case. On January 12, 2024, the Court agreed to stay this case pending resolution of the Ciarciello case.
On February 22, 2021,9, 2024, another plaintiff filed a derivative shareholder lawsuit against certain of the Company’s current and former directors and officers (in which the Company finalized allis a nominal defendant) filed in the United States District Court for the District of Delaware, Sanderson, on behalf of Bioventus Inc., v. Reali, et.al., No. 1:24-cv-00180-RGA (D. Del. 2024). Like the Grogan case, this case asserts violations of Section 10(b) of the Exchange Act, breaches of fiduciary duties and related state law claims, and a claim for contribution, and generally alleges the same purported misconduct as alleged in the Ciarciello case. The parties are in discussions to consolidate the two derivative matters and stay them on terms relatedsimilar to those entered in the settlement and entered into a formal settlement agreement with the USAO and OIG consistent with the previous agreement in principle and which included releases from associated False Claims Act liability and further Civil Monetary Penalties that are customary in self-disclosures of this type and resolved the potential liability related to the Company’s self-disclosure for $3,600, of which $2,400 had previously been paid. The remaining $1,200 net settlement amount due under the agreement was recorded in accrued liabilities within the consolidated balance sheets as of December 31, 2020 and paid on February 23, 2021. Refer to Note 17. Subsequent events for further details regarding the resolution.
Reserve for estimated overpayments from all third-party payersGrogan case.
The Company maintainsbelieves the claims alleged in each of the above matters lack merit and intends to defend itself vigorously. The outcome of these matters is not presently determinable, and any loss is neither probable nor reasonably estimable.
Misonix former distributor litigation
On March 23, 2017, Misonix’s former distributor in China, Cicel (Beijing) Science & Technology Co., Ltd., filed a reservelawsuit against Misonix and certain of its officers and directors in the United States District Court for reimbursementthe Eastern District of New York. The complaint alleged that Misonix improperly terminated its contract with the former distributor. The complaint sought various remedies, including compensatory and punitive damages, specific performance and preliminary and post judgment injunctive relief, and asserted various causes of action, including breach of contract, unfair competition, tortious interference with contract, fraudulent inducement, and conversion. On October 7, 2017, the court granted Misonix’s motion to dismiss each of the tort claims relatedasserted against Misonix, and also granted the individual defendants’ motion to dismiss all claims asserted against them. On January 23, 2020, the court granted Cicel’s motion to amend its Minimally Invasive Fracture Treatment product that may have been processedcomplaint, to include claims for paymentalleged defamation and theft of trade secrets in addition to the breach of contract claim. Discovery in the matter ended on August 5, 2021. On January 20, 2022, the court granted Misonix’s summary judgment motion on Cicel’s breach of contract and defamation claims. Cicel’s motion for reconsideration of the court’s summary judgment ruling in Misonix’s favor was dismissed by the Court on April 29, 2022. On July 18, 2022, Cicel voluntarily dismissed the remaining claim for trade secret theft and later filed an appeal to the United States Court of Appeals for the Second Circuit. On March 6, 2024, the Second Circuit Court of Appeals issued its ruling affirming the lower Court’s summary judgment in favor of Misonix in all respects.
Bioness stockholder litigation
On February 8, 2022, a minority shareholder of Bioness filed an action in the Delaware State Court of Chancery in connection with the Company’s acquisition of Bioness, Teuza, a Fairchild Technology Venture Ltd. v. Lindon, et. al., No. 2022-0130 -SG. This action names the former Bioness directors, the Alfred E. Mann Trust (Trust), which was the former majority shareholder of Bioness, the trustees of the Trust and Bioventus as defendants. The complaint alleges, among other things, that the individual directors, the Trust, and the trustees breached their fiduciary duty to the plaintiff in connection with their consideration and approval of the Company’s transaction. The complaint also alleges that the Company without adequate medical records support.aided and abetted the other defendants in breaching their fiduciary duties to the plaintiff and that the Company breached the Merger Agreement by failing to pay the plaintiff its pro rata share of the merger consideration. The Company held a reserve of $2,790 and $6,801 at December 31, 2020 and 2019, respectivelybelieves that it is indemnified under the indemnification provisions contained in the Bioness Merger Agreement for these amounts. Theclaims. On July 20, 2022, the Company refunded Medicare $1,519 and $7,458 duringfiled a motion to dismiss all claims made against it on various grounds, as did all the years ended December 31, 2020 and 2019, respectively, related to known and estimated overpayments for medical necessity included in this reserve for periods through December 31, 2019. Certain of these overpayments were identified as potential overpaymentsother named defendants in the Company’s OIG self-disclosure in November 2018. The Company’s reservesuit. A hearing on Bioness’ and other the defendant’s motions was estimated using extrapolationheld before the Court of Chancery on January 19, 2023. On April 27, 2023, the Court issued an error rateorder which, among other things, dismissed Bioventus from a statistical sample, which represents the Company’s best estimate as of the date of the financial statements, but because of the uncertainty inherent in such estimates, the ultimate resolution may be materially different.
Product recall
In December 2020, we voluntarily recalled our ultrasound gel, an accessory to the Minimally Invasive Fracture Treatment product. We have incurred, and expect to incur in the future, costs associated with this recall. Based on the information that has been received, we have estimated the probable loss related to this recall globally to be approximately $1,684. We have recorded reserves representing the probable loss within accrued liabilities on the consolidated balance sheet. The final outcome of this recall is dependent on many factors that are difficult to predict.case.
Other matters
On August 23, 2019,November 10, 2021, the Company and Harbor entered into an exclusive collaborationasset purchase agreement for purposesa hyaluronic acid (“HA”) product and made an upfront payment of developing a product for orthopedic uses to be commercialized by the Company and supplied by Harbor. As part of the agreement a third-party license was assigned to us and$853. If the Company is subjectable to obtain a 3% royalty on certain commercial sales, or a nominal minimum amount per quarter, beginning in 2023.Medical Device Regulation Certification for the product $1,707 (the “Milestone Payment”) will be paid to the seller within five days. The Company is obligatedalso required to pay up to $6,000 upon achieving certain milestones. Unless earlier terminated,royalties if certifications are achieved before December 31, 2024. Royalties will be payable through 2026 of 5.0% on the first $569 in sales and 2.5% thereafter. On March 8, 2023, the parties amended the agreement will remain in effect untilunder which the earlierMilestone Payment was reduced to $1,418, of 8 years or untilwhich $709 was paid on January 31, 2024, and the paymentremainder is due upon receipt of certain milestones are met.
the Medical Device Regulation Certification for the product provided that is obtained prior to December 31, 2024. As a result, the Company recorded an intellectual property intangible asset totaling $709 for the initial payment.On May 29, 2019, the Company and the Musculoskeletal Transplant Foundation, Inc. d/b/a MTF Biologics (MTF)(“MTF”), entered into a collaboration and development agreement to develop one or more products for orthopedic application to be commercialized by the Company and supplied by MTF.MTF (the “Development Agreement”). The first phase has been completed. Additional fees forcompleted, but during the subsequent phases will be determined assecond quarter of 2022, the Company elected to discontinue the development work progresses. Theof MOTYS, the initial product candidate under development. On October 21, 2022, the Company provided notice to MTF of termination of the Development Agreement continues untiland the date when the parties execute a supply agreementrelated cGTP Commercial Supply Agreement with MTF for the commercial products.MOTYS, effective December 20, 2022.
On December 9, 2016, the Company entered into an amended and restated license agreement for the exclusive U.S. distribution and commercialization rights of a single injection OAosteoarthritis (“OA”) product with the supplier of the Company’s single injection OA product for the non-U.S. market. The agreement requires the Company to meet annual minimum purchase requirements and pay royalties on net sales. Royalties related to this agreement totaled $10,021, $7,622 and $3,082 forduring the years ended December 31, 2020, 20192023, 2022 and 2018 respectively,2021 totaled $14,035, $14,712 and $13,300, respectively. These royalties are included in cost of sales onwithin the consolidated statementstatements of operations and comprehensive income (loss). income.
As part of a supply agreement entered on February 9, 2016 and subsequently amended for the Company’s three injection OA product, the Company is subject to annual minimum purchase requirements for ten10 years. After the initial ten10 years, the agreement will automatically renew for an additional five5 years unless terminated by the Company or the seller in accordance with the agreement.
As part of a supply agreement for the Company’s five injection OA product that was amended and restated on December 22, 2020, the Company is subject to annual minimum purchase requirements for eight8 years.
The Company has an exclusive license agreement for bioactive bone graft putty. The Company is required to pay a royalty on all commercial sales revenue from the licenselicensed products with a minimum annual royalty payment through 2023, the date the agreement will expire, upon the expiration of the patent held by the licensor. These royalties are included in cost of sales on the consolidated statementstatements of operations and comprehensive income (loss). income.
From time to time, the Company causes LOCsletters of credit (“LOCs”) to be issued to provide credit support for guarantees, contractual commitments and insurance policies. The fair values of the LOCs reflect the amount of the underlying obligation and are subject to fees payable to the issuers, competitively determined in the marketplace. As of December 31, 20202023 the Company had three LOCs outstanding for $1,800 and 2019,as of December 31, 2022, the Company had one LOC outstanding for a nominal amount.
The Company currently maintains insurance for risks associated with the operation of its business, provision of professional services and ownership of property. These policies provide coverage for a variety of potential losses, including loss or damage to property, bodily injury, general commercial liability, professional errors and omissions and medical malpractice. The Company is self-insured for health insurance covering most of its employees located in the United States. The Company maintains stop-loss insurance on a “claims made” basis for expenses in excess of $150$200 per member per year.
In the normal course of business, the Company periodically becomes involved in various claims and lawsuits, and governmental proceedings and investigations that are incidental to the business. With respect to governmental proceedings and investigations, like other companies in our industry, the Company is subject to extensive regulation by national, state and local governmental agencies in the U.S. and in other jurisdictions in which the Company and its affiliates operate. As a result, interaction with governmental agencies is ongoing. The Company’s standard practice is to cooperate with regulators and investigators in responding to inquiries. The outcomes of legal actions are not within the Company’s complete control and may not be known for extended periods of time. Other than the matters discussed above, management of the Company, after consultation with legal counsel, does not believe there are any unrecorded matters that will have a material adverse effect upon the Company’s financial statements.
13. Net income (loss) per unit
The following table presents the computation of basic and diluted net income (loss) per unit for the years ended December 31 as follows:
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Net income from continuing operations | $ | 14,722 | | | $ | 8,113 | | | $ | 4,443 | |
| Loss attributable to noncontrolling interest | 1,689 | | | 553 | | | — | |
| $ | 16,411 | | | $ | 8,666 | | | $ | 4,443 | |
| | | | | |
| Net income from continuing operations attributable to unit holders | $ | 16,411 | | | $ | 8,666 | | | $ | 4,443 | |
| Accumulated and unpaid preferred distributions | (6,133) | | | (5,955) | | | (5,781) | |
| Net income allocated to participating shareholders | (5,895) | | | (1,555) | | | — | |
Net income (loss) from continuing operations attributable to
common unit holders | 4,383 | | | 1,156 | | | (1,338) | |
| Loss from discontinued operations, net of tax | — | | | 1,815 | | | 16,650 | |
| Net income (loss) attributable to common unit holders | $ | 4,383 | | | $ | (659) | | | $ | (17,988) | |
| | | | | |
Net income (loss) per unit attributable to common unit holders
- basic and diluted | | | | | |
| Net income (loss) from continuing operations | $ | 0.89 | | | $ | 0.24 | | | $ | (0.27) | |
| Loss from discontinued operations, net of tax | — | | | 0.37 | | | 3.40 | |
| Net income (loss) attributable to common unit holders | $ | 0.89 | | | $ | (0.13) | | | $ | (3.67) | |
| | | | | |
| Weighted average common units outstanding, basic and diluted | 4,900,000 | | | 4,900,000 | | | 4,900,000 | |
The computation of diluted earnings per unit for the years ended December 31, 2020, 2019 and 2018 excludes the effect of the 6,590 potential common units that would be issued upon the conversion of preferred units. The effect of these units would be antidilutive due to the impact of the accumulated and unpaid preferred distributions as well as the Company being in a net loss position for the years ended December 31, 2019 and 2018.
14. Net SalesRevenue recognition
The Company attributes net sales to external customers to the U.S. and to all foreign countries based on the locationlegal entity from which the sale originated. The following table presents ourthe Company’s net sales by segment disaggregated by geographic markets and major products (Vertical)within each segment as follows for the years ended December 31 as follows:31:
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Primary geographic markets: | | | | | |
| U.S. | $ | 293,697 | | | $ | 305,072 | | | $ | 282,895 | |
| International | 27,464 | | | 35,069 | | | 36,282 | |
| Total net sales | $ | 321,161 | | | $ | 340,141 | | | $ | 319,177 | |
| Vertical: | | | | | |
| OA joint pain treatment and joint preservation | $ | 171,178 | | | $ | 182,082 | | | $ | 155,576 | |
| Minimally invasive fracture treatment | 88,624 | | | 103,504 | | | 121,032 | |
| Bone graft substitutes | 61,359 | | | 54,555 | | | 42,569 | |
| Total net sales | $ | 321,161 | | | $ | 340,141 | | | $ | 319,177 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | 2023 | | 2022 | | 2021 |
| U.S. | | | | | | | | | |
| Pain Treatments | | | | | $ | 197,954 | | | $ | 194,830 | | | $ | 201,068 | |
| Restorative Therapies | | | | | 116,851 | | | 134,214 | | | 103,009 | |
| Surgical Solutions | | | | | 135,055 | | | 126,207 | | | 83,476 | |
| Total U.S. net sales | | | | | 449,860 | | | 455,251 | | | 387,553 | |
| | | | | | | | | |
| International | | | | | | | | | |
| Pain Treatments | | | | | 22,847 | | | 21,495 | | | 20,539 | |
| Restorative Therapies | | | | | 20,222 | | | 20,420 | | | 18,563 | |
| Surgical Solutions | | | | | 19,416 | | | 14,951 | | | 4,243 | |
| Total International net sales | | | | | 62,485 | | | 56,866 | | | 43,345 | |
| | | | | | | | | |
| Total net sales | | | | | $ | 512,345 | | | $ | 512,117 | | | $ | 430,898 | |
15.14. Segments
SegmentThe Company’s two reportable segments are U.S. and International. The Company’s products are primarily sold to orthopedists, musculoskeletal and sports medicine physicians, podiatrists, neurosurgeons and orthopedic spine surgeons, as well as to their patients. The Company does not disclose segment information by asset, is not disclosednor does it disclose transactions between reportable segments, as it is not reviewed by the CODM does not review or useduse it to allocate resources or to assess the operating results and financial performance. We believe EBITDA, adjusted for additional non-operational factors disclosedTransactions between reportable segments are executed in accordance with our transfer pricing arrangements and are eliminated in consolidation and not presented in the table below, or Adjustedmeasures below. Segment adjusted EBITDA is a key measure for internal reporting. Adjusted EBITDA should not be considered in isolation or as a substitute for consolidated net income (loss)attributablethe segment profitability metric reported to the Company, the most closely analogous U.S. GAAP measure. Adjusted EBITDA is not defined in the same manner by all companiesCompany’s CODM for purposes of decisions about allocation of resources to, and may not be comparable to other similarly titled measuresassessing performance of, other companies unless the definition is the same. each reportable segment.
The following table presents segment adjusted EBITDA reconciled to (loss) income from continuing operations before income taxes for the years ended December 31 as follows:31:
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Segment adjusted EBITDA from continuing operations | | | | | |
| U.S. | $ | 69,252 | | | $ | 71,673 | | | $ | 67,480 | |
| International | 3,191 | | | 7,515 | | | 4,691 | |
| Depreciation and amortization | (28,643) | | | (30,316) | | | (29,238) | |
| Interest expense | (9,751) | | | (21,579) | | | (19,171) | |
| Equity compensation | (10,103) | | | (10,844) | | | (14,325) | |
| COVID-19 benefits, net | 4,123 | | | — | | | — | |
| Succession and transition charges | (5,609) | | | — | | | — | |
| Restructuring costs | (563) | | | (575) | | | (1,373) | |
| Foreign currency impact | 117 | | | (8) | | | (234) | |
| Equity loss in unconsolidated investments | (467) | | | — | | | — | |
| Other non-recurring costs | (5,633) | | | (6,177) | | | (1,723) | |
| Income from continuing operations before income taxes | $ | 15,914 | | | $ | 9,689 | | | $ | 6,107 | |
| | | | | | | | | | | | | | | | | |
| 2023 | | 2022 | | 2021 |
| Segment adjusted EBITDA | | | | | |
| U.S. | $ | 78,668 | | | $ | 56,513 | | | $ | 70,712 | |
| International | 10,194 | | | 12,097 | | | 9,915 | |
| Interest expense, net | (40,676) | | | (12,021) | | | (1,112) | |
| Depreciation and amortization | (57,365) | | | (55,398) | | | (34,875) | |
| Acquisition and related costs | (5,694) | | | (21,731) | | | (22,964) | |
| Restructuring and succession charges | (2,331) | | | (7,453) | | | (3,717) | |
| Equity compensation | (2,722) | | | (17,585) | | | 4,512 | |
| Financial restructuring costs | (7,291) | | | — | | | — | |
| Impairments of assets | (78,615) | | | (10,285) | | | — | |
| Impairment of goodwill | — | | | (124,697) | | | — | |
| Loss on disposal of a business | (1,539) | | | — | | | — | |
| Impairments related to variable interest entity | — | | — | | (7,043) | |
| Other items | (13,740) | | | (8,465) | | | (5,940) | |
| (Loss) income before income taxes | $ | (121,111) | | | $ | (189,025) | | | $ | 9,488 | |
16.15. Discontinued operations
In December 2018,On February 27, 2023 the Company underreached a Settlement Agreement with the directionFormer Securityholders of CartiHeal that resulted in the transfer of 100% of Company’s shares in CartiHeal to a Trustee. Refer to Note 4. Acquisitions and authority ofdivestitures for further details concerning the CartiHeal Settlement Agreement and its deconsolidation from the Company’s Board of Managers, committed to shut down the bone morphogenetic protein (BMP) research and development program, whichfinancial statements. CartiHeal had been reported as its own segment in previous years. Substantially all operations, including project close documentation, contract termination, vacating the facility and ultimately the termination of the employees, ceased by March 2019 and as a result the BMP research and development program met the criteriano sales for discontinued operations. The Company sold the remaining $172 held for sale asset and paid the remaining $400 accrued liability from the BMP research and development program during the year ended December 31, 2020.2022 and December 31, 2023.
The following table summarizes CartiHeal’s major classes of assets and liabilities as reported on the consolidated balance sheets as of December 31, 2022 as the balances were fully deconsolidated as of December 31, 2023:
| | | | | |
| December 31, 2022 |
| Carrying amounts of major classes of assets included as part of discontinued operations | |
| Cash | $ | 1,628 | |
| Restricted cash | 23 | |
| Other receivables | 350 | |
| Inventory | 642 | |
| Prepaid and other current assets | 134 | |
| Current assets attributable to discontinued operations | 2,777 | |
| Property and equipment, net | 191 | |
| Goodwill | 6,297 | |
| Intangible assets, net | 398,873 | |
| Operating lease assets | 618 | |
| Other assets | 15 | |
| Long-term assets attributable to discontinued operations | 405,994 | |
| Total assets attributable to discontinued operations | $ | 408,771 | |
| Carrying amounts of major liabilities included as part of discontinued operations | |
| Accounts payable | $ | 852 | |
| Accrued liabilities | 384 | |
| Current portion of deferred consideration | 117,615 | |
| Other current liabilities | 236 | |
| Current liabilities attributable to discontinued operations | 119,087 | |
| Deferred income taxes | 79,863 | |
| Deferred consideration | 79,269 | |
| Contingent consideration | 67,251 | |
| Other long-term liabilities | 2,528 | |
| Long-term liabilities attributable to discontinued operations | 228,911 | |
| Total liabilities attributable to discontinued operations | $ | 347,998 | |
The following table summarizes the statementmajor income and expense line items of these discontinued operations, and comprehensive income (loss) from discontinuedas reported in the consolidated statements of operations for the years ended December 31:
| | | | | | | | | | | |
| 2019 | | 2018 |
| Research and development expense | $ | 1,773 | | | $ | 7,127 | |
| Loss on disposal | 52 | | | 9,638 | |
| Income tax benefit | (10) | | | (115) | |
| Loss from discontinued operations, net of tax | $ | 1,815 | | | $ | 16,650 | |
| | | | | | | | | | | | | | | | | |
| 2023 | | 2022 | | 2021 |
| | | | | |
| | | | | |
| | | | | |
| Selling, general and administrative expense | $ | 1,728 | | | $ | 472 | | | $ | — | |
| Research and development expense | 396 | | | 2,087 | | | — | |
Change in fair value of contingent consideration(a) | 1,710 | | | 5,350 | | | — | |
Depreciation and amortization(a) | 4,264 | | | 11,405 | | | — | |
| Impairments of assets | — | | | 64,500 | | | — | |
| Operating loss from discontinued operations | (8,098) | | | (83,814) | | | — | |
Interest expense, net(a) | 4,889 | | | 13,774 | | | — | |
Other expense (income)(b) | 61,442 | | | (22,714) | | | 1,868 | |
| Other expense (income) | 66,331 | | | (8,940) | | | 1,868 | |
| Loss before income taxes | (74,429) | | | (74,874) | | | (1,868) | |
| Income tax benefit, net | — | | | (6,134) | | | — | |
| Net loss from discontinued operations | (74,429) | | | (68,740) | | | (1,868) | |
Loss attributable to noncontrolling interest —
discontinued operations | 14,937 | | | 13,955 | | | — | |
Net loss attributable to Bioventus Inc. —
discontinued operations | $ | (59,492) | | | $ | (54,785) | | | $ | (1,868) | |
(a)Depreciation and amortization, the change in fair value of contingent consideration and interest expense represent the significant operating non-cash items of discontinued operations.
(b)Other expense includes the $60,639 loss on deconsolidation, of which $10,150 was attributable to non-refundable payments. Total investing cash outflows included these non-refundable payments and $1,356 cash on hand at disposal.
17.16. Subsequent events
Investment
On January 4, 2021,As disclosed in Note 5. Financial Instruments, the Company made a convertible debt investmentamended the 2019 Credit Agreement on January 18, 2024 to further modify certain financial covenants applicable to borrowings under the 2019 Credit Agreement. In addition, during the period commencing on the Closing Date and ending upon the satisfaction of $1,500 in a medical device company.
Recapitalization
On February 16, 2021, the following Transactions occurred.
•The Company amended and restated its limited liability company agreement (New LLC Agreement)certain conditions occurring not prior to among other things, (i) provide for the new single class of common membership interests inOctober 29, 2025, the Company will be subject to certain additional financial covenants and requirements as discussedset forth in Note 1. Organization and basis of presentation for financial information; (ii) exchange all of the then existing membership interests of the common and preferred unit holders (Original LLC Owners) for Common LLC Interests and (ii) appoint the New LLC Owner as the sole managing member of the Company. The amendment resulted in the Company’s common and preferred units, including the preferred distribution rights, being converted into 47,625,326 Common LLC Interests.
•The New LLC Owner amended and restated its certificate of incorporation to, among other things, (i) provide for Class A common stock and Class B common stock, each share of which entitles its holders to one vote per share on all matters presented to the New LLC Owner’s stockholders and (ii) issue 15,786,737 shares of Class B common stock to the Continuing LLC Owner, on a one-to-one basis with the number of LLC Interests it owns. While the Class B common stock holds voting rights it holds no economic interest in the New LLC Owner.
•The New LLC Owner acquired, by merger, the Former LLC Owners and upon consummation of the merger, owned 31,838,589 Common LLC Interests.
•The New LLC Owner closed an IPO of 9,200,000 shares of Class A common stock at a public offering price of $13.00 per share. The New LLC Owner received $111,228 in proceeds, net of underwriting discounts and commissions, which was used to make a capital contribution to the Company in exchange for 9,200,000 Common LLC Interests of the Company at a price per interest equal to the IPO price of $13.00.
Subsequent to the Transactions, the New LLC Owner owns 41,038,589 Common LLC Interests or 72.2% of the Company and the Continuing LLC Owner owns 15,786,737 or 27.8%. The New LLC Owner has a majority economic interest, the sole voting interest in, and controls the management of the Company. As a result, the New LLC Owner will consolidate the financial results of the Company and will report a non-controlling interest representing the interests owned by the Continuing LLC Owner.
The New LLC Agreement requires that the Company, at all times, maintain (i) a one-to-one ratio between the number of shares of Class A common stock issued by the New LLC Owner and the number of Common LLC Interests owned by the New LLC Owner and (ii) a one-to-one ratio between the number of shares of Class B common stock owned by the Continuing LLC Owner and the number of LLC Interests owned by the Continuing LLC Owner.
On February 22, 2021, the Company finalized all terms related to a settlement agreement with the USAO and the OIG with respect to the submission of Medicare claims that did not meet CMS coverage requirements and for which our sales representatives completed Section B of the CMN forms. The Company entered into a formal settlement agreement with the USAO and OIG which included releases from associated False Claims Act liability and further Civil Monetary Penalties that are customary in self-disclosures of this type and resolved the potential liability related to the Company’s self-disclosure in this matter for $3,600, of which $2,400 has already been paid through the Company’s 2019 return of overpayments described previously. The remaining $1,200 net settlement amount due under the agreement was recorded in accrued liabilities within the consolidated balance sheets as of December 31, 2020 and was paid on February 23, 2021.5. Financial instruments.
Item 9.Changes in and Disagreements Withwith Accountants on Accounting and Financial Disclosures.
None.
Item 9A.Controls and Procedures.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our President and Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report.Report on Form 10-K. Based on this evaluation, our President and Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2020.2023.
Management’s Report on Internal Control over Financial Reporting
This Annual Report on Form 10-K does not include a report of management's assessment regardingThe Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting or an attestation(as such term is defined in the Exchange Act Rule 13a-15(f)). The Company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of our independent registered public accounting firm due to a transition period established by rulesrecords that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the SEC for newly public companies. assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In addition, because we areconnection with the preparation and filing of this Annual Report, the Company’s management, including our President and Chief Executive Officer and our Chief Financial Officer, conducted an "emerging growth company" under the JOBS Act, our independent registered public accounting firm will not be required to attest toevaluation of the effectiveness of our internal control over financial reporting as of December 31, 2023, based on the framework set forth in “Internal Control—Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on the Company’s evaluation, our President and Chief Executive Officer and Chief Financial Officer, concluded that, as of December 31, 2023, the Company’s internal controls over financial reporting were effective.
Material Weaknesses in Internal Control over Financial Reporting
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected and corrected on a timely basis.
Changes in Control Environment
As previously reported, we identified a material weakness in internal control related to an ineffective risk assessment in 2022 to identify and assess changes in our business processes and internal control environment related to newly acquired companies and multiple information technology (“IT”) system implementations that occurred. Further, there was a lack of adequate personnel resources in accounting, IT, and other support functions to support simultaneous system implementations and business process integrations for so longacquired companies, implement appropriate controls for acquired companies and maintain focus on compliance with internal controls for legacy Bioventus processes.
In addition, during 2022, the Company saw an unprecedented level of turnover in roles that drive execution of internal control activities. This turnover, paired with business changes, including those related to acquisitions, resulted in a disruption to the effective completion of control activities across a number of business processes. Further, management identified a gap in control design related to sufficient tracking of control performance to ensure controls operated effectively.
In considering these breakdowns in the control environment, Bioventus determined the associated COSO principles requiring further control and action by management were:
(a)Control environment - Establishes structure, authority, and responsibility (COSO Principle 3);
(b)Risk assessment – Identifies and analyzes significant change (COSO Principle 9); and
(c)Monitoring – Conducts ongoing and /or separate evaluations (COSO Principle 16).
These ongoing control deficiencies resulted in certain immaterial restatements of the Company’s financial statements as discussed in our Quarterly Reports on Form 10-Q for the periods ended April 1, 2023, July 1, 2023 and September 30, 2023.
Remediation Measures
During 2023, management implemented our previously disclosed remediation plans including:
(a)Ensuring adequate personnel were in place, taking steps to decrease turnover and driving additional accountability, including: (i) hiring personnel and temporary resources to backfill positions vacated due to employee turnover and adding additional headcount in accounting, finance and IT to expand capacity;(ii) making organizational changes to ensure proper governance and oversight; (iii) implementing certain retention programs to retain key resources; (iv) driving additional accountability for control owners by tying a portion of their performance objectives to successful completion of internal controls;
(b)Enhancing our risk assessment processes to (i) identify and assess changes for acquired companies and IT systems; (ii) prioritize key projects to ensure organizational capacity, with only essential IT projects occurring during 2023; (iii)update our internal control policy that further defines expectations for communication and approval of changes to financially relevant processes and internal controls.
(c)Enhancing tracking of control performance and reinforcing execution rigor by (i) establishing and monitoring monthly internal control metrics compared with target performance; (ii) establishing required quarterly internal control certifications; (iii) providing additional visibility of internal control performance to management and the Audit Committee; (iv) engaging third-party consultants to perform an Accounting Transformation & Integration Assessment (“Project Action”) that enhanced processes and use of existing systems for financially relevant processes which allow for more time for internal control execution; (vi) meeting weekly with leadership to discuss actions needed to successfully close internal control deficiencies identified; (v) updating and/or developing standard operating procedures to further document process and control performance for use in day-to-day execution and when training new employees.
During the fourth quarter of 2023, we completed our testing of the operating effectiveness of the implemented and remediated controls and found them to be effective. As a result, we have concluded the material weakness has been remediated as of December 31, 2023.
Rebates Accrual Material Weakness
As previously reported, we identified a material weakness related to the Company’s internal controls over financial reporting that were not performed at a sufficient level of precision to ensure that the third quarter 2022 rebates accrual was complete and accurate. The process undertaken to estimate the expected reduction in revenue from rebates was consistent with the Company’s historical practice. However, subsequent to the initial calculation of the third quarter 2022 rebates accrual, an unexpectedly large invoice was received and there were not processes in place to ensure it was reviewed timely in order to update the accrual.
The Company reassessed open rebates accruals and the approach for calculating the rebate accruals based on this invoice. The Company revised its estimation methodology resulting in a decrease of revenue of $8.4 million. This adjustment was recorded subsequent to the earnings release but prior to the filing of the Company’s Quarterly Report on Form 10-Q for the third quarter of 2022. Further, this change in revenue projection related to the rebates accrual adjustment for 2022 and cascading effect on future revenue projections materially impacted the Company’s evaluation of its ability to meet debt covenants in its Amended 2019 Credit Agreement, resulting in liquidity and going concern disclosures in the Company’s Quarterly Report on Form 10-Q for the third quarter of 2022.
Remediation Measures
We have designed and implemented new processes and enhanced controls to address the underlying causes of the material weakness related to the rebates accrual, including:
•Refining the estimation method for calculating the rebates accruals, including enhancing the precision of the controls;
•Implementing enhanced status tracking and introduced new controls to ensure that rebates invoices from third-party payers are an emerging growth company.received and reviewed timely; and
•Increasing rigor of documenting key conversations with payers.
The Company implemented and enhanced the precision of procedures to ensure the completeness and accuracy of key reports and information used in the rebates accrual and further enhanced supporting documentation for control performance.
During the fourth quarter of 2023, we completed our testing of the operating effectiveness of the enhanced controls and found them to be effective. As a result, we have concluded the material weakness has been remediated as of December 31, 2023.
We believe the implementations and continued operation of the actions described above with respect to our control environment and rebates accrual processes are sufficient to remediate our previously identified material weaknesses and strengthen our internal control over financial reporting.
When considered in the aggregate, the previously identified material weaknesses no longer create a reasonable possibility that a material misstatement to the consolidated financial statements would not be prevented or detected on a timely basis. Therefore, management concluded that the deficiencies no longer represent a material weakness in our internal control over financial reporting, and that disclosure controls and procedures were effective, at the reasonable assurance level, as of December 31, 2023.
The President and Chief Executive Officer and Chief Financial Officer believe that the financial statements and related financial information included in this Annual Report on Form 10-K fairly present, in all material respects, our balance sheets, statements of operations and comprehensive (loss) income, statement of changes in stockholders’ equity and statements of cash flows as of and for the periods presented.
Changes in Internal Control over Financial Reporting
During 2020 we remediated a material weakness associated with the proper processing of Exogen reimbursement claims in accordance with regulations and contractual terms.
We implemented the following measures to improve our internal controls and remediate the material weakness:
•the augmentation, reorganization and training of our prescription to cash staff, which includes our direct sales team, order management personnel, patient financial services personnel and reimbursement services and accounts receivable personnel, regarding key aspects of regulations and requirements and how to deal with inconsistencies within patient medical records;
•implementation of monthly sales order testing on sampling basis by our Compliance department including a review of medical necessity;
•establishment of a cross functional governance committee, reporting to an executive steering committee to review and approve our Exogen Medicare policy and oversee future Exogen policy and process interpretations and changes; and
•implementation of a checklist to be completed for each Medicare order to ensure compliance with our policy for Medicare claims and then further automating this checklist.
Other than the changes described above, thereThere were no other changes toin our internal control over financial reporting that occurred during the fourth quarter ended December 31, 2020of 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.reporting except for changes to controls resulting from the material weaknesses described above.
Attestation Report of Independent Registered Public Accounting Firm
This Annual Report does not include an attestation report on our internal control over financial reporting of our registered public accounting firm due to an exemption established by the JOBS Act for emerging growth companies.
Item 9B.Other Information.
During the quarter ended December 31, 2023, none of our directors or officers (as defined in rule 16a-1(f) under the Exchange Act) adopted, modified or terminated a “Rule 10b5-1 trading arrangement” or a “non-Rule 10b5-1 trading arrangement” (as such terms are defined in Item 408 of Regulation S-K).
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.Applicable.
PART III
Item 10.Directors, Executive Officers and Corporate Governance.
MANAGEMENT
Executive officers, key employees and directors
The following table sets forth the name, age and position(s) of each ofinformation regarding our directors and executive officers as of March 15, 2021:1, 2024:
| | | | | | | | | | | | | | |
| Name | | Age | | Position(s) |
Executive OfficersRobert E. Claypoole | | 52 | | | |
Kenneth M. Reali | | 55 | | President, Chief Executive Officer and Director |
Gregory O. Anglum | | 50 | | Senior Vice President and Chief Financial Officer |
John E. Nosenzo | | 63 | | Chief Commercial Officer |
Anthony D’Adamio | | 60 | | Senior Vice President and General Counsel |
Katrina Church | | 59 | | Senior Vice President and Chief Compliance Officer |
Alessandra Pavesio | | 54 | | Senior Vice President and Chief Science Officer |
Non-Employee Directors | | | | |
William A. Hawkins(4) | | 70 | | 67 | Director, Chairperson |
John A. Bartholdson | | 53 | | Director |
| Patrick J. Beyer | | 58 | | Director |
Philip G. Cowdy(1)(3) | | 56 | | 53 | Director |
Mary Kay Ladone | | 57 | | Director |
| Michelle McMurry-Heath | | 54 | | Director |
Guido J. Neels(2) | | 75 | | 72 | Director |
Guy P. Nohra(2)(3) | | 63 | | 60 | Director |
David J. Parker(1)
| | 60 | | Director |
Martin P. Sutter(3) | | 68 | | 65 | Director |
Susan M. Stalnecker(1)(4) | | 71 | | 68 | Director |
(1) Member of the audit and risk committee
(2) Member of the compensation committee
(3) Member of the nominating and corporate governance committee
(4) Member of the compliance, ethics and culture committee
Kenneth M. Reali has servedRobert Claypoole joined Bioventus as our Chief Executive Officer since April 2020 and as a member of our board of directors (Board) since September 2020. Mr. Reali previously served as President, and Chief Executive Officer of Clinical Innovations, LLC, a medical device company focused on advancing woman’s healthcare, from June 2015 until its successful sale on February 12, 2020. In this role, Mr. Reali led the company through two successful acquisitions by a private equity firm in October 2017 and later to a leading diagnostic and therapeutic medical technology company in February 2020. Prior to joining Clinical Innovations, LLC, Mr. Reali also served as the President and CEO of Baxano Surgical, Inc., a medical device company, from January 2010 until February 2015, leading its turn-around out of bankruptcy. Mr. Reali also held positions of increasing responsibility at several medical device companies, including Biomet, Inc. (now known as Zimmer Biomet) and Stryker Corporation. Mr. Reali also served as Senior Vice President and General Manager within the Biologics and Clinical Therapies business of Smith & Nephew plc from May 2005 to January 2010, a division which was later spun out to become Bioventus LLC (BV LLC). Mr. Reali has served as a member of the board of managers of BV LLC since April 2020. Mr. Reali also currently serves as a member of the board of directors of Ossio, Ltd., an orthopedic medical device company, the Advanced Medical Technology Association, or AdvaMed, an American medical device trade association, and DYSIS Medical Ltd., a medical device company focused on the noninvasive, in-vivo detection of cancerous and pre-cancerous lesions. Mr. Reali also serves on the compensation committee of Ossio, Ltd. and the ethics and health care compliance committee of AdvaMed. Mr. Reali holds a Bachelor of Science in Business Administration from Valparaiso University. We believe Mr. Reali is qualified to serve on our Board because of his vast skills and experience in the medical device industry, his role as our Chief Executive Officer and his extensive knowledgea director in January 2024. Please see Mr. Claypoole’s biography set forth in Part I, Item 1. Business—Information about our Executive Officers of the Company.this Annual Report.
Gregory O. Anglum has served as our Senior Vice President and Chief Financial Officer since May 2017. Previously, Mr. Anglum served as our Chief Accounting Officer from April 2016 to May 2017. Prior to joining us, Mr. Anglum served as Chief Financial Officer of Overture Networks, Inc. (now known as ADVA Optical Networking SE), a leading global provider of networking and telecommunications equipment from September 2015 to April 2016. From December 2014 to September 2015, Mr. Anglum was Chief Financial Officer at StrikeIron, Inc., a Data-as-a-Service software company. From August 2004 to July 2014, Mr. Anglum was an audit partner at Grant Thornton LLP, or GT, where he also served as leader of the Raleigh office from August 2009 through July 2014 and was a member of the firm-wide leadership team for the technology industry group. Mr. Anglum holds a Master of Business Administration with a concentration in Accounting from Vanderbilt University’s Owen Graduate School of Management and a Bachelors of Arts in Economics from Vanderbilt University and is a Certified Public Accountant.
John E. Nosenzo has served as our Chief Commercial Officer since February 2017. Prior to joining us, Mr. Nosenzo served as Senior Vice President, Global Customer Operations at Beckman Coulter Diagnostics, a global leader in clinical diagnostics, from September 2011 to February 2017. From May 2010 to September 2011, Mr. Nosenzo was Senior Vice President, Customer Relations Management for Siemens Healthcare (now known as Siemens Healthineers AG), a clinical diagnostic services and therapeutic systems company, where he developed and implemented sales plans for their multi-billion dollar healthcare imaging and healthcare IT commercial organizations. Mr. Nosenzo’s earlier career also includes senior positions at Quest Diagnostics and Bayer Healthcare LLC’s Diagnostics Division (now known as Siemens Healthcare Diagnostics). Mr. Nosenzo currently serves as a member of the board of directors of Spectral Medical Inc. Mr. Nosenzo holds a Master of Business Administration in marketing and management from Adelphi University and received his Bachelor of Science in pharmacy from St. John’s University.
Anthony D’Adamio has served as our Senior Vice President and General Counsel since August 2017. Previously, Mr. D’Adamio was General Counsel and Secretary at Siemens Healthcare (now known as Siemens Healthineers AG) from January 2010 to August 2017 and served as Deputy General Counsel and Secretary of Siemens Healthcare Diagnostics from January 2007 to January 2010. Prior to that, Mr. D’Adamio was Senior Counsel within the Diagnostics Division of Bayer Healthcare LLC (now known as Siemens Healthcare Diagnostics) from January 2001 to December 2006. Mr. D’Adamio began his legal career at the law firm of Bond, Schoeneck & King before taking corporate legal positions with companies within the health insurance, pharmaceutical and biotechnology industries, including Group Health Incorporated, Quest Diagnostics and Covance Inc. Mr. D’Adamio holds a Juris Doctor, cum laude, from Howard University School of Law and a Bachelor of Arts from the State University of New York at Binghamton.
Katrina Church has served as our Chief Compliance Officer since August 2020. Prior to joining us, Ms. Church served in corporate counsel and compliance roles within the Merz Group of companies, most recently as Global Compliance Officer for Merz Pharma GmbH & Co KGaA, a privately-held pharmaceutical company, from March 2015 to August 2020. From June 1998 to December 2008, Ms. Church was Executive Vice President and General Counsel of Connetics Corporation, a specialty pharmaceutical company that was acquired by Stiefel Laboratories, Inc. in 2008. Ms. Church began her career as an attorney at Hopkins & Carley, a San Jose-based law firm. In 2020, Ms. Church was nominated for several industry awards for compliance training and received the 2020 Women in Compliance Award for “Most Impactful Compliance Training Programme of the Year” and the Brandon Hall 2020 Gold Medal for Excellence in Training. Ms. Church holds a Juris Doctor from New York University School of Law and a Bachelor of Arts in Comparative Literature, magna cum laude, from Duke University.
Alessandra Pavesio has served as our Senior Vice President and Chief Science Officer since August 2013. Previously, Ms. Pavesio managed the Boston University Coulter Translational Partnership, a foundation-sponsored research program designed to enhance clinical impact and wealth creation through the development and transfer of innovative intellectual properties from university laboratories to commercial practice, from January 2012 to July 2013. From January 2010 to December 2011, Ms. Pavesio was Vice President of Research & Development at Anika Therapeutics, Inc., an integrated orthopedic medicines company. Prior to that, Ms. Pavesio served as Director of Research and Development at Fidia Advanced Biopolymers, s.r.l. (now known as Anika Therapeutics, Inc.), from May 1991 to December 2009. Ms. Pavesio is the co-author of numerous peer reviewed publications and more than 15 patented inventions on hyaluronan based and biologics technologies. In the European Union, she has also served as chairperson of international regenerative medicine technology platforms and government advisory councils on innovation. Ms. Pavesio holds a Master’s degree in Medicinal Chemistry, magna cum laude, from the University of Turin in Italy.
William A. Hawkins has served as a member of our Board since September 2020 and as Chairperson of our Board since September 2020. Mr. Hawkins is a Senior Advisor to EW Healthcare Partners, a leading private equity firm investing in life sciences. From October 2011 to July 2015, Mr. Hawkins served as President and Chief Executive Officer of Immucor, Inc., a leading provider of transfusion and transplantation diagnostic products worldwide. Prior to that, Mr. Hawkins served in positions of increasing responsibility at Medtronic, Inc., a prominent medical technology company, from January 20022001 to June 2011, most recently serving as its Chief Executive Officer from November 2007 to June 2011. Mr. Hawkins served as President and Chief Executive Officer of Novoste Corporation, a global leader in the field of vascular brachytherapy, from 1988 to 2002 and has also held several senior leadership positions at American Home Products (now known as Wyeth, LLC), Johnson & Johnson, Guidant Corp. and Eli Lilly and Co. Mr. Hawkins has served as a member of the board of managers of BV LLC sincefrom January 2016.2016 until the time of our IPO. Mr. Hawkins also currently serves on the board of directors of Avanos Medical, Inc., a public medical technology company; Biogen Inc. and MiMedx Group Inc., each a public biopharmaceutical company; and Asklepios BioPharmaceutical, Inc., Baebies, Inc., Cirtec Medical Corp., Immucor, Inc.Enterra Medical and Virtue Labs, LLC, each a privately-held life science company. Mr. Hawkins was electedserves on the compensation committee of Biogen and chairs the ethics and compliance committee of MiMedx. Mr. Hawkins previously served on the Board of Directors of Avanos Medical, Inc. from 2015 to April 2021 and Immunor, Inc. from 2015 to 2023. Mr. Hawkins served on the Duke University Board of Trustees infrom 2011 to 2023, where he held the position of Vice Chair, and currently serves as its Vice Chairman.was recently appointed Duke University Trustee Emeritus. Mr. Hawkins is also previously served as the Chair of the Duke University Health System board of director anddirectors. He is currently a member of the board of directors of the North Carolina Biotechnology Center and the Focused Ultrasound Foundation Society. Mr. Hawkins holds a Master of Business Administration from the University of Virginia Darden School of Business and received a Bachelor of Science in electrical and biomedical engineering from Duke University. Mr. Hawkins was selected to serve on our Board because of his experience in and knowledge of the life science industry.
John A. Bartholdson was appointed as a member of our Board in January 2023 and his appointment became effective January 8, 2023. Mr. Bartholdson is the co-founder and has been a Partner of Juniper Investment Company, a private investment management firm that invests in publicly traded and private companies through concentrated ownership positions since its inception in 2007. Mr. Bartholdson has 25 years of experience leading and overseeing private and public equity investments. His experience includes extensive management oversight, service on multiple public and private company boards, and deep transactional expertise. Mr. Bartholdson presently serves as the Chairman of the board of directors of Theragenics Corporation, a medical device company serving the surgical products and prostate cancer treatment markets. From 2019, he has been a member of the board of directors of Lincoln Educational Services Corporation, a public company and a leading provider of career education and training services, and presently serves on its Compensation, Audit and Nominating and Corporate Governance Committees. Previously, he served as a member of the board of directors of Obagi Medical Products, Inc., a public specialty pharmaceutical company, until its acquisition by Valeant Pharmaceuticals in 2013. In addition, Mr. Bartholdson has previously served on the board of directors of numerous private companies. Mr. Bartholdson was a Partner of Stonington Partners, where he worked from 1997 to 2011. Prior to that, he was an analyst at Merrill Lynch Capital Partners from 1992 to 1994. Mr. Bartholdson received his Bachelor of Arts from Duke University and his Master of Business Administration from Stanford Graduate School of Business. Mr. Bartholdson was selected to serve on our board because of his significant governance, finance, and transactional experience on multiple public and private company boards.
Patrick J. Beyer has served as a member of our Board since October 2021. Mr. Beyer is the President of International and Global Orthopedics for ConMed Corporation, a publicly held medical technology company, a position in which he has served since October 2020. He previously served as President of ConMed International from December 2014 to October 2020. Prior to joining ConMed, Mr. Beyer served as Chief Executive Officer of ICNet, a privately held infectious control software company from 2010 to 2014 when the company was sold. Prior to this, he spent 21 years at Stryker Corporation where he led Stryker Europe from 2005 to 2009; Stryker UK, South Africa and Ireland from 2002 to 2005 and Stryker Medical from 1999 to 2002. Mr. Beyer previously served on the board of directors of Misonix, Inc. from May 2021 to October 2021, where he was a member of its audit committee. Mr. Beyer graduated from Kalamazoo College with a Bachelor of Arts in Economics, Western Michigan University with a Master of Business Administration in Finance and Harvard Business School’s Advanced Management Program. Mr. Beyer was selected to serve on our Board because of his extensive healthcare and public company experience.
Philip G. Cowdy has served as a member of our Board since September 2020. Mr. Cowdy ishas served as the Chief Business Development and Corporate Affairs Officer for Smith & Nephew plc.plc, a medical equipment manufacturing company, since 2018 Since joining Smith & Nephew plc in June 2008, he has also served as Executive Vice President of Business Development and Corporate Affairs, Head of Corporate Affairs and Strategic Planning, Group Director of Corporate Affairs and Director of Investor Relations. Prior to joining Smith & Nephew plc, Mr. Cowdy served as a Senior Director at Deutsche Bank for 13 years, providing corporate finance and equity capital markets advice to a variety of UK-based companies. Mr. Cowdy is currentlyserved as a member of the board of managers of BV LLC which he has served on from January 2012 to October 2017 and again from July 2018 until the time of our IPO, and he has served as a member of its Audit, Compliance and Quality Committee. Mr. Cowdy received his Bachelor of Science in Natural Sciences from Durham University (UK)(“UK”) and is a qualified chartered accountant. Mr. Cowdy was selected to serve on our Board because of his experience in the industry, his finance experience, and his knowledge of the Company.
Mary Kay Ladone has served as a member of our Board since July 2021. Ms. Ladone served as Senior Vice President, Corporate Development, Strategy and Investor Relations, of Hill-Rom Holdings, Inc. (“Hill-Rom”), a medical technology provider, from December 2018 to December 2021. Ms. Ladone previously served as Hill-Rom’s Vice President, Investor Relations, July 2016 to December 2018. Ms. Ladone served as Senior Vice President, Investor Relations, of Baxalta Inc. from 2015 to 2016 before joining Hill-Rom. Prior to Baxalta Inc., Ms. Ladone served in a variety of senior finance, business development and investor relations roles for Baxter International, Inc. Since March 2022, Ms. Ladone has also served on the board of directors of Inogen Inc., a publicly traded supplemental oxygen therapies provider, where she is a member of the audit and compensation committees. Ms. Ladone also serves on the board of directors of Kestra Medical Technologies, Inc., a privately held wearable medical device and digital healthcare company, where she has been the chair of the audit committee since September 2022. Ms. Ladone holds a Bachelor of Arts in Finance and Economics from the University of Notre Dame. Ms. Ladone was selected to serve on our Board due to her significant finance and investor relations experience at large healthcare companies.
Michelle McMurry-Heath, MD, PhD, has served as a member of our Board since January 2022. Dr. McMurry-Heath served as President and Chief Operating Officer of the Biotechnology Innovation Organization, a membership and advocacy organization focused on improving biotech research and applying biotech innovations to major healthcare challenges, from 2020 to 2022. Dr. McMurry-Health was previously with Johnson & Johnson (“J&J”) from 2014 to 2020, where she served as Global Head of Evidence Generation for Medical Device Companies and then Vice President of Global External Innovation and Global Leader for Regulatory Sciences. Prior to her time at J&J, Dr. McMurry-Heath was a key science policy leader in government, conducting a comprehensive analysis of the National Science Foundation’s policies, programs and personnel. President Obama then named her associate science director of the FDA’s Center for Devices and Radiological Health where she served from 2010 to 2014. From 2005 to 2010, Dr. McMurry-Heath was Director of the Health, Biomedical Science and Society Policy Program at the Aspen Institute. Dr. McMurry-Heath began her career as a Senior Policy Advisor for Senator Joseph Lieberman for Health, Social, and Biomedical Innovation Policy from 2001 to 2004. She later served as a Robert Wood Johnson Health and Society Scholar at the University of California, San Francisco and Berkeley from 2004 to 2005 and a Mcarthur Fellow, Global Health for the Council on Foreign Relations from 2004 to 2006. Dr. McMurry-Heath also serves on the Board of Directors at publicly traded PerkinElmer, where she is a member of the audit committee. Dr. McMurray-Heath received her M.D./Ph.D. in Immunology from Duke University’s Medical Scientist Training Program, becoming the first African American to graduate from the prestigious program, and her AB in Biochemistry from Harvard University. Dr. McMurry-Heath was selected to serve on our Board due to her significant policy, regulatory, commercial health care and advocacy experience.
Guido J. Neels has served as a member of our Board since September 2020. Mr. Neels has been with EW Healthcare Partners (formerly Essex WoodlandsWoodlands), a healthcare growth equity and venture capital firm, since August 2006, where he is now anhas served as Operating Partner.Partner since 2013. Prior to joining Essex Woodlands,EW Healthcare Partners, Mr. Neels served in a variety of management positions at Guidant Corporation, a developer of cardiovascular medical products. From July 2004 until retiring in November 2005, Mr. Neels served as Guidant’s Chief Operating Officer, where he was responsible for the global operations of Guidant’s four operating units: Cardiac Rhythm Management, Vascular Intervention, Cardiac Surgery and Endovascular Solutions. From December 2002 to July 2004, Mr. Neels served as Guidant’s Group Chairman, Office of the President, responsible for worldwide sales operations, corporate communications, corporate marketing, investor relations and government relations. In January 2000, Mr. Neels was named Guidant’s President, Europe, Middle East, Africa and Canada. In addition, Mr. Neels served as Guidant’s Vice President, Global Marketing, Vascular Intervention, from 1996 to 2000 and as Guidant’s General Manager, Germany and Central Europe, from 1994 to 1996. Mr. Neels has served as a member of the board of managers of BV LLC sincefrom May 2012.2012 until the time of our IPO. Mr. Neels also currently serves on the board of directors of Axogen, Inc. and also is a member of its compensation committee. Mr. Neels previously served on the board of directors of Endologix, Inc. from December 2010 to June 2019 and on the board of directors of Entellus Medical from November 2009 to February 2018, each of which is a public company. Mr. Neels holds a Master in Business Administration from the Stanford University Graduate School of Business and received his Business Engineering degree from the University of Leuven in Belgium. Mr. Neels was selected to serve on our Board because of his experience in the industry, familiarity with serving on the boards of public companies and his knowledge of our business.
Guy P. Nohra has served as a member of our Board since September 2020. In March 1996, Mr. Nohra co-founded Alta Partners, a life sciences venture capital firm, and he has since been involved in the funding and development of numerous medical technology and life sciences companies. Mr. Nohra is currentlyserved as a member of the board of managers of BV LLC, which he has served on sincefrom May 2012 and serves asuntil the Chairtime of the Compensation Committee.our IPO. Mr. Nohra currently serves as a member of the boards of directors of severalSpiral Therapeutics, Inc., a private life sciences companies, including Bionure, Inc., Sanifit Therapeutics S.A. and Spiral Therapeutics, Inc.company. He also previously served on the board of directors of various public companies, including ATS Medical, Inc., Cutera, Inc., AcelRx Pharmaceuticals, Inc., and ZS Pharma, as well as several private companies, including Bionure, Inc., Sanifit Therapeutics S.A., Carbylan Biosurgery, Inc., Cerenis Therapeutics, Coapt Systems, Paracor Medical, Inc. and PneumRx. Mr. Nohra holds a Master in Business Administration from the University of Chicago and received his Bachelor of Arts in History from Stanford University. Mr. Nohra was selected to serve on our Board because of his extensive experience in the life sciences industry, his investment and development experience, and his service as a director of other life sciences companies.
David J. Parker has served as a member of our Board since September 2020. Mr. Parker has been a General Partner at Ampersand Capital Partners, a private equity firm with $800.0 million of capital under management, since November 2010, which he originally joined in September 1994. Prior to joining Ampersand Capital Partners, Mr. Parker served as a management consultant at Bain & Company and Mercer Management Consulting, where he provided strategy and operations consulting services to clients in the healthcare, transportation, consumer products and telecommunications sectors. Mr. Parker is currently a member of the board of managers of BV LLC, which he has served on since May 2012, and serves on its Audit, Compliance and Quality Committee. Mr. Parker also currently serves as a director of Genome Diagnostics B.V., or GenDx, MedPharm Ltd. and Tjoapack Holdings B.V. Mr. Parker also serves on the remuneration committees of both GenDx and MedPharm and the audit and remediation committees of GenDx. Mr. Parker holds a Master of Business Administration in Finance from The Wharton School of the University of Pennsylvania and received his Bachelor of Arts in Government and Economics from Dartmouth College. Mr. Parker was selected to serve on our Board because of his extensive experience in the life sciences industry, his finance and investment experience, and his service as a director of other life sciences companies.
Martin P. Sutter has served as a member of our Board since September 2020. Mr. Sutter is one of the two founding Managing Directors of EW Healthcare Partners (previously known as Essex Woodlands), one of the oldest and largest life sciences and healthcare focused growth equity and venture capital firms, which he formed in 1994.1985. Mr. Sutter has more than 3035 years of management experience in operations, marketing, finance and venture capital. Mr. Sutter has served as a member of the board of managers of BV LLC sincefrom May 2012.2012 until the time of our IPO. Mr. Sutter also currently serves on the board of directors of Abiomed, Inc., a publicly traded biopharmaceutical company, MiMedx Group, Inc., a publicly traded medical devicesregenerative medicine life sciences company, and Prolacta Biosciences, Inc., a privately held life sciences company. Mr. Sutter has also previously served on the board of directors of Abiomed, Inc., Tissue Tech, Inc., and Suneva Medical, Inc. and QSpex Technologies, Inc. Mr. Sutter currently serves on the compensation and nominating and governance committees of both Abiomed,MiMedx Group, Inc. and MiMedx Group,Prolacta Biosciences, Inc. and previously served on the compensation and nominating and governance committee of Abiomed, Inc. Mr. Sutter holds a Master of Business Administration from the University of Houston and received his Bachelor of Science from Louisiana State University. Mr. Sutter was selected to serve on our Board because of his extensive experience in the life sciences industry, his investment experience, and his service as a director of other life sciences companies.
Susan M. Stalnecker has served as a member of our Board since September 2020. Ms. Stalnecker has been a Senior Advisor at Boston Consulting Group, a global management consulting firm, since March 2016. Ms. Stalnecker served as Vice President of E.I. duPont de Nemours and Co. (now known as DuPont de Nemours, Inc., or DuPont), a public company, a diversified science and innovations public company and leader in the fields of healthcare, electronics and transportation, from December 1976 until she retired in 2016. During her nearly 40-year career at DuPont, Ms. Stalnecker served in several senior leadership roles including Vice President, Treasurer & M&A; Vice President, Risk Management; Vice President, Government and Consumer Markets; and Vice President, Productivity & Shared Services. Ms. Stalnecker has served as a member of the board of managers of BV LLC sincefrom November 2018.2018 until the time of our IPO. Ms. Stalnecker also currently serves on the board of directors of Leidos Holding, Inc. and Optimum Funds McQuairie, and serves on the Board of Trustees of the Duke Health System.McQuairie. She also serves on the audit & finance committee of Leidos Inc., and the audit committee of Optimum Funds McQuairie andMcQuairie. From 2009 to 2023, Ms. Stalnecker served on the compliance, audit & finance committeesBoard of Trustees of the Duke Health System.System, where she was a member of the compliance, audit and finance committees. Ms. Stalnecker holds a Master of Business Administration from The Wharton School of the University of Pennsylvania and received her Bachelor of Arts from Duke University. Ms. Stalnecker was selected to serve on our Board because of her extensive experience as a financial expert, her investment experience, and her service as a director of other public companies.
AuditAdditional information required by this Item concerning our directors is incorporated by reference from the sections captioned “Election of Directors,” “Corporate Governance” and Risk Committee and audit committee financial expert
We have a separately designated standing Audit and Risk committee which consists“Committees of Philip G. Cowdy, David J. Parker and Susan M. Stalnecker, with Susan M. Stalnecker serving as chair. We intendthe Board” contained in our definitive proxy statement for all membersour 2024 Annual Meeting of our Audit and Risk CommitteeStockholders to meet the definition of “independent director” for purposes of serving on the Audit and Risk Committee by February 11, 2022, in accordancebe filed with the phase-in rulesSEC within 120 days after the end of Rule 10A-3 under the Securities Exchange Act of 1934, as amended (Exchange Act) and the rules of fiscal year ended December 31, 2023.
The Nasdaq Stock Market LLC (Nasdaq) requiring that the Audit and Risk Committee be composed entirely of members who qualify as independent. We have determined that the fact thatinformation required by this Item concerning our Audit and Risk Committee is not be entirely comprisedincorporated by reference from the section captioned “Committees of independent directors in the first year following our IPO will not materially adversely affect the ability of our AuditBoard-Audit and Risk CommitteeCommittee” contained in our definitive proxy statement for our 2024 Annual Meeting of Stockholders to act independently and to satisfybe filed with the other requirementsSEC within 120 days after the end of the SEC and Nasdaq. Our Board has affirmatively determined that David J. Parker and Susan M. Stalnecker meet the definition of “independent director” for purposes of serving on an Audit and Risk Committee under Rule 10A-3 and the Nasdaq rules, and we intend to comply with the other independence requirements within the time periods specified. In addition, our Board has determined that Susan M. Stalnecker qualifies as an “audit committee financial expert,” as such term is defined in Item 407(d)(5)(ii) of Regulation S-K.
fiscal year ended December 31, 2023.Code of compliance and ethics
We have adopted a written code of compliance and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A current copy of the code is posted on our website, www.bioventus.com. In addition, we intend to post on our website all disclosures that are required by law or Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the code.
The information onrequired by this Item concerning our websiteexecutive officers is deemed not to be incorporated in this Annual Report or to be partset forth at the end of Part I of this Annual Report on Form 10-K.Report.
The information required by this Item, if any, concerning compliance with Section 16(a) of the Exchange Act will be incorporated by reference from the section captioned “Delinquent Section 16(a) Reports” contained in our definitive proxy statement for our 2024 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2023.
Item 11.Executive Compensation.
ThisThe information required by this Item is incorporated by reference from the section discussescaptioned “Executive and Director Compensation” contained in our definitive proxy statement for our 2024 Annual Meeting of Stockholders to be filed with the material componentsSEC within 120 days after the end of the executive compensation program for our executive officers who are named in the “2020 Summary compensation table” below. In 2020, our “named executive officers” and their positions were as follows:
•Kenneth M. Reali, Chief Executive Officer;
•Gregory O. Anglum, Senior Vice President & Chief Financial Officer;
•John E. Nosenzo, Senior Vice President & Chief Commercial Officer;
•Anthony D’Adamio, Senior Vice President & General Counsel;
•Alessandra Pavesio, Senior Vice President & Chief Science Officer; and
•Anthony P. Bihl III, former Chief Executive Officer.
Mr. Reali became Chief Executive Officer and a member of the BV LLC board of managers, effective as of April 13, 2020, in connection with Mr. Bihl’s retirement on April 19, 2020.
2020 Summary Compensation Table
The following table sets forth information concerning the compensation of our named executive officers for thefiscal year ended December 31, 2019 and 2020.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and Principal Position | | Year | | Salary ($)(1) | | Bonus ($) | | Stock Awards ($)(7) | | Option Awards ($)(8) | | Non-Equity Incentive Plan Compensation ($)(5) | | All Other Compensation ($)(6) | Total ($) |
| Kenneth M. Reali................................... | | 2020 | | $ | 428,135 | | | $ | 9,282 | | (2) | $ | 4,111,191 | | | $ | 38,631 | | | $ | 426,634 | | | $ | 277,719 | | $ | 5,291,592 | |
| Chief Executive Officer | | 2019 | | — | | | — | | | — | | | — | | | — | | | — | | — | |
| Gregory O. Anglum.......................... | | 2020 | | 381,044 | | | 2,793 | | (2) | — | | | — | | | 178,471 | | | 22,392 | | 584,700 | |
| Senior Vice President & Chief Financial Officer | | 2019 | | 374,019 | | | — | | | — | | | — | | | 143,479 | | | 22,386 | | 539,884 | |
| John E. Nosenzo | | 2020 | | 504,893 | | | 21,778 | | (3) | — | | | — | | | 357,646 | | | 22,959 | | 907,276 | |
| Senior Vice President & Chief Commercial Officer | | 2019 | | 490,219 | | | — | | | — | | | — | | | 295,322 | | | 22,584 | | 808,125 | |
| Anthony D’Adamio | | 2020 | | 396,597 | | | 3,062 | | (2) | — | | | — | | | 195,674 | | | 22,959 | | 618,292 | |
| Senior Vice President & General Counsel | | 2019 | | 391,106 | | | — | | | — | | | — | | | 150,016 | | | 22,584 | | 563,706 | |
| Alessandra Pavesio | | 2020 | | 408,657 | | | 3,267 | | (4) | — | | | — | | | 201,652 | | | 21,375 | | 634,951 | |
| Senior Vice President & Chief Science Officer | | 2019 | | 398,165 | | | — | | | — | | | — | | | 160,693 | | | 21,000 | | 579,858 | |
| Anthony P Bihl III | | 2020 | | 236,750 | | | — | | | — | | | — | | | 214,111 | | | 3,639,984 | | 4,090,845 | |
| Former Chief Executive Officer | | 2019 | | 684,979 | | | — | | | — | | | — | | | 552,910 | | | 23,561 | | 1,261,450 | |
___________
(1)Amounts reflect annual base salary earned with respect to 2019 and 2020.
(2)Amounts reflect discretionary “make whole” bonuses paid to all employees who earned performance-based cash incentives under the 2020 Annual Incentive Plan in amounts equal to the additional cash incentives that would have otherwise been paid under such plan had salaries not been reduced in connection with the COVID-19 pandemic, as described below under “—2020 Salaries”.
(3) Amount reflects a discretionary “make whole” bonus paid to Mr. Nosenzo in respect of his performance-based cash incentive under the 2020 Executive Annual Incentive Plan – Chief Commercial Officer in an amount equal to (i) the additional cash incentive that he would have otherwise been paid under such plan had his salary not been reduced in connection with the COVID-19 pandemic, as described below under “—2020 Salaries” and (ii) in recognition of his sales efforts during the COVID-19 pandemic, the additional cash incentive that he would have been paid had he been a participant in the 2020 Executive Annual Incentive Plan – Non Commercial.
(4)Amount reflects (i) a $3,167 discretionary “make whole” bonus paid to Ms. Pavesio in respect of her performance-based cash incentive under the 2020 Executive Annual Incentive Plan – Non Commercial in an amount equal to the additional cash incentive that she would have otherwise been paid under such plan had her salary not been reduced in connection with the COVID-19 pandemic, as described below under “—2020 Salaries”, and (ii) $100 bonus paid to Ms. Pavesio in connection with a CEO determination to pay $100 discretionary bonuses to all employees with five through nine years of service.
(5)Amounts reflect the annual performance-based cash incentives earned by our named executive officers in 2019 and 2020 based on achievement of corporate and personal performance objectives as set forth in the 2019 and 2020 Executive Annual Incentive Plan – Non Commercial or the 2019 and 2020 Executive Annual Incentive Plan – Chief Commercial Officer, as applicable.
(6)2020 amounts reflect (i) $8,550, $8,550, $8,550, $8,550, $8,550, and $8,550 in matching 401(k) contributions made by us to the 401(k) accounts of Messrs. Reali, Anglum, Nosenzo, D’Adamio, Ms. Pavesio and Mr. Bihl, respectively, (ii) additional fixed non-elective contributions of $1,064, $12,402, $12,825, $12,825, $12,825, and $12,825 made by us to the 401(k) accounts of Messrs. Reali, Anglum, Nosenzo, D’Adamio, Ms. Pavesio and Mr. Bihl, respectively, (iii) reimbursement of cellular telephone expenses to Messrs. Reali, Anglum, Nosenzo, and D’Adamio equal to $1,056, $1440, $1,584 and $1,584, respectively, (iv) relocation allowance of $250,000 and tax gross up of $17,048 to Mr. Reali, (v) payments to Mr. Bihl of a $4,966 benefit stipend and $63,700 tax stipend in connection with his status as a partner and associated receipt of a guaranteed payment instead of a salary, and (vi) payments made to Mr. Bihl in connection with his retirement in an aggregate amount of $3,549,943 to reflect the COVID-19-related decrease in the repurchase value of his equity interests.
2019 amounts reflect (i) $2,561 benefit stipend to Mr. Bihl (ii) $8,400, $8,400, $8,400, $8,400 and $8,400 in matching 401(k) contributions made by us to the 401(k) accounts of Messrs. Anglum, Nosenzo, D’Adamio, Ms. Pavesio and Mr. Bihl, respectively, (iii) additional fixed non-elective contributions of $12,600, $12,600, $12,600, $12,600 and $12,600 made by us to the 401(k) accounts of Messrs. Anglum, Nosenzo, D’Adamio, Ms. Pavesio and Mr. Bihl, respectively, and (iv) reimbursement of cellular telephone expenses to Messrs. Anglum, Nosenzo, and D’Adamio equal to $1,386, $1,584 and $1,584, respectively.
(7)Amount reflects the aggregate grant date fair value of phantom profits interests granted to Mr. Reali during the year ended December 31, 2020 computed in accordance with FASB ASC Topic 718, Compensation—Stock Compensation. Refer to Note 9. Equity-based compensation within Part II, Item 8. Financial Statements and Supplementary Data for a discussion of the relevant assumptions used in calculating this amount.
(8)Amount reflects the aggregate grant date fair value of options granted to Mr. Reali during the year ended December 31, 2020 computed using a Black-Scholes calculation in accordance with FASB ASC Topic 718, Compensation—Stock Compensation.
Narrative to Summary Compensation Table
Employment Letters
The terms of employment for Messrs. Reali, Anglum, Nosenzo, D’Adamio and Ms. Pavesio in effect as of December 31, 2020 are documented in their employment letters dated March 12, 2020, August 2, 2017, November 18, 2016, July 11, 2017 and June 13, 2013, respectively, with Mr. Reali’s employment letter subsequently amended as of April 24, 2020. The terms of Mr. Bihl’s prior employment are documented in his offer letter dated November 4, 2013 as amended on October 17, 2019 to reflect his status as a partner. Pursuant to their respective employment letters, Mr. Bihl was hired to serve as the Chief Executive Officer, Mr. Reali was hired to serve as the Chief Executive Officer following Mr. Bihl’s retirement, Mr. Anglum was promoted on August 7, 2017 to serve as the Chief Financial Officer (after serving as the interim Chief Financial Officer effective May 1, 2017), Mr. Nosenzo was hired to serve as Chief Commercial Officer, Mr. D’Adamio was hired to serve as Senior Vice President and General Counsel and Ms. Pavesio was hired to serve as Chief Science Officer. Mr. Bihl also served as a member of the BV LLC board of managers until his retirement on April 19, 2020 and Mr. Reali now serves as a member of the BV LLC board of managers. In connection with our IPO, we entered into new employment agreements with Messrs. Reali, Anglum, Nosenzo, D’Adamio, and Ms. Pavesio (as further described below under “—Severance”).
2020 Salaries
The named executive officers were entitled to receive a base salary in 2020 to compensate them for services rendered to us. The base salary payable to each named executive officer is intended to provide a fixed component of compensation reflecting the named executive officer’s skill set, experience, role and responsibilities. The annual base salaries payable to Messrs. Reali, Anglum, Nosenzo, D’Adamio and Ms. Pavesio as of December 31, 2020, were $615,000, $389,881, $518,451, $405,794 and $419,630 respectively, all of which reflect merit increases which were effective March 29, 2020 for the named executive officers other than Mr. Reali. In connection with the COVID-19 pandemic, we implemented a 20% reduction in base salary for each of our employees with a base salary of $100,000 or greater, including each of our named executive officers, effective May 31, 2020 and ending June 27, 2020, which reductions are reflected in the actual salary paid in the summary compensation table.
Effective as of the consummation of our IPO, we increased the salaries of Messrs. Reali, Anglum, D’Adamio and Ms. Pavesio to $700,000, $430,000, $420,000 and $430,000, respectively.
2020 Incentive Bonuses
With respect to their services in 2020, Messrs. Reali, Anglum, D’Adamio, Ms. Pavesio and Mr. Bihl were eligible to earn an annual performance-based cash bonus pursuant to the 2020 Executive Annual Incentive Plan – Non Commercial, or the Non Commercial AIP (with Mr. Bihl’s bonus pro-rated for the portion of the year for which he provided service prior to his retirement date), and Mr. Nosenzo was eligible to earn an annual performance-based cash bonus pursuant to the 2020 Executive Annual Incentive Plan – Chief Commercial Officer, or the Commercial AIP. Bonuses earned by our named executive officers under both the Non Commercial AIP and Commercial AIP were based upon weighted minimum, target and maximum achievement of both business and personal performance measures. The Non Commercial AIP and the Commercial AIP objective business measures in 2020 were (1) Global Revenue, (2) Adjusted Global EBITDA and (3) multiple osteoarthritis treatment achievements, including submission of an IND application to FDA for “Biologic” placental tissue product in the third quarter of 2020, the launch of a GTP placental tissue product in the fourth quarter of 2020 and the achievement of $100,000 in 2020 revenue with respect to such placental tissue product. The personal performance standards were based on the named executive officers’ performance ratings.
Mr. Reali’s and Mr. Bihl’s target incentive for 2020 was 100% of their respective eligible earnings (as defined under the applicable AIP); Mr. Nosenzo’s was 75% of his eligible earnings; and Messrs. Anglum, D’Adamio and Ms. Pavesio’s was 50% of their respective eligible earnings.
Objective business measures and personal performance were weighted as 80% and 20%, respectively, of the annual bonuses under the Non Commercial AIP and the Commercial AIP. Possible payouts for the objective business measures under the Non Commercial AIP and the Commercial AIP ranged from 50% for minimum achievement, 100% for target achievement, to 200% for maximum achievement, which amounts shall correspond to 95%, 100%, and 105% achievement of the applicable objective business measure target, respectively. The personal performance component of the award amount ranged from a 50% for minimum achievement, 100% for target achievement, to 200% for maximum achievement.
For 2020, the achievement percentage for the objective business measures for each of our NEOs was determined to be 93.6% (with Mr. Bihl’s pro-rated award calculated at actual achievement for the objective business measures). The achievement percentage for the personal performance component was determined to be 125% for each of Messrs. Reali, D’Adamio, Nosenzo, and Ms. Pavesio and 100% for Mr. Anglum (with Mr. Bihl’s pro-rated award calculated at target achievement (100%) for the personal performance component). In addition to the awards calculated under the applicable AIP for each of our NEOs, each of our employees who participated in an AIP, including our NEOs, received a discretionary “make whole” bonus with respect to each employee’s performance-based cash incentive under the applicable AIP in an amount equal to the additional cash incentive each employee would have otherwise received had salaries not been reduced in connection with the COVID-19 pandemic. In recognition of his sales efforts during the COVID-19 pandemic, Mr. Nosenzo also received an additional discretionary “make whole” bonus with respect to his performance-based cash incentive under the Commercial AIP in an amount equal to the additional cash incentive he would have received had he been a participant in the Non Commercial AIP.
Equity-Based Compensation
Prior to our IPO, we maintained the BV LLC profits interest plan, which we called the Management Incentive Plan (MIP), pursuant to which we granted 333,330 profits interest units of BV LLC (Profits Interest Units), to Mr. Bihl on December 2, 2013. In connection with Mr. Bihl’s retirement, we redeemed all of his Profits Interest Units as described below under “—Severance.” In connection with our IPO the MIP was terminated and no further awards will be made under the MIP.
Prior to our IPO, we also maintained the Bioventus Phantom Profits Interest Plan, which was renamed the Bioventus Stock Plan on June 1, 2020, and which we called the Phantom Plan, pursuant to which we granted time-vesting phantom plan units (Time Phantom Units) and performance-vesting phantom plan units (Performance Phantom Units). The Time Phantom Units generally vested ratably over five years (20% on the first anniversary of the date of grant and 5% quarterly thereafter) and entitled the holder to a cash payment, in an amount determined by reference to the value of our Profits Interest Units, with respect to any vested Time Phantom Units upon the earlier of a termination from service or certain distribution events with respect to the Company’s profits interest units. In the event of a qualifying distribution event prior to a termination, all Time Phantom Units fully vest. Generally, Performance Phantom Units were scheduled to vest on June 1, 2021, subject to the achievement of 2020 corporate revenue goals, but could also become vested in whole or in part in the board of managers’ discretion in the event such revenue goals were not satisfied. In connection with our IPO, on February 11, 2021, we terminated the Phantom Plan and there will be no further awards made under the Phantom Plan. We will settle all awards thereunder 12 months following the termination. In connection with the Phantom Plan termination, Bioventus Inc. assumed the obligations of BV LLC and Phantom Plan awards will be paid in the form of shares of Class A common stock (or, in the case of former employees whose employment terminated prior to the offering and who hold vested Phantom Plan awards as of 12 months following the termination of the Phantom Plan, in the form of cash). The number of shares of Class A common stock received by each participant, including our named executive officers, were determined by dividing (A) the value of the participant’s vested Phantom Units (after giving effect to any accelerations in vesting in connection with our IPO, as described below) as of February 11, 2021 by (B) the $13.00 IPO price of our Class A common stock (with any terminated employees holding vested Phantom Plan awards receiving the cash value of such shares determined as of the date of the IPO). It is anticipated that, to the extent that a Time Phantom Unit is not otherwise vested as of the date the Phantom Plan is terminated, settlement with respect to such Time Phantom Unit will be subject to the holder’s continued employment with us through the applicable vesting date or the twelve month anniversary of plan termination, if earlier. As a result of the $13.00 IPO price of our Class A common stock, the Performance Phantom Units had no value as the IPO date.
Each of our named executive officers holds Phantom Plan awards in BV LLC as set forth below in “—Outstanding Equity Awards at Fiscal-Year End.” On June 25, 2020, in connection with the commencement of his employment with us, Mr. Reali was granted 417,804 Phantom Units. In addition, on July 30, 2020, Mr. Reali was granted an option to purchase up to 5,935 equity interests of BV LLC at a per unit price of $42.12 at any time prior to July 30, 2021 (or the termination of his service, if earlier), but Mr. Reali forfeited this option prior to our IPO. No other named executive officers received a grant of equity or phantom equity awards in 2020.
New Equity-Based Compensation
In connection with our IPO, our Board adopted the 2021 Incentive Award Plan (2021 Incentive Plan), in order to facilitate the grant of cash and equity incentives to our non-employee directors, employees (including our named executive officers) and consultants and employees and consultants of our subsidiaries and to enable our Company and our subsidiaries to obtain and retain the services of these individuals, which is essential to our long-term success. In connection with our IPO, we granted the following under the 2021 Incentive Plan to certain of our employees and non-employee directors, including the named executive officers:
•options to purchase 4,561,500 shares of Class A common stock that vest between two and four years from February 11, 2021 and
•restricted stock unit (RSU) awards for 293,170 and 67,500 to employees and non-employee directors, respectively, of shares of Class A common stock released between one and four years from February 11, 2021
In connection with our IPO, our Board also adopted the 2021 Employee Stock Purchase Plan (ESPP).
Severance
The employment letters in effect as of December 31, 2020 for each of our named executive officers provide for severance payments upon termination of employment by us at any time without cause (other than as a result of death or disability) or a termination by the named executive officer for good reason (as defined below)during the two year period following the date of a change in control (as defined in the respective employment letter). In the event of a termination by us without cause, each of our named executive officers would be entitled to receive (1) twelve months’ base salary, payable in a lump sum within 60 days following termination of employment, (2) 100% of their respective target annual cash incentive, payable in a lump sum within 60 days following termination of employment, and (3) payment of COBRA premiums for the first twelve months of coverage following termination of employment. In the event of a termination by Messrs. Anglum, Nosenzo, D’Adamio and Ms. Pavesio for good reason during the two-year period following a change in control, Messrs. Anglum, Nosenzo, D’Adamio and Ms. Pavesio would be entitled to receive the same severance payments and benefits as in the case of termination by us without cause. In the event of a termination by Mr. Reali for good reason during the two-year period following a change in control, under his employment letter Mr. Reali is entitled to receive enhanced severance equal to 18 months of each of his base salary and his target annual cash incentive, each payable in a lump sum on or about 60 days following termination of employment, as well as payment of COBRA premiums for the first 18 months of coverage following termination of employment. The severance payments are conditioned upon execution and delivery of a release and compliance with confidentiality and restrictive covenant obligations as set forth in a separate proprietary information agreement.
In connection with his retirement on April 19, 2020, Mr. Bihl was not entitled to receive any severance or other benefits under his employment letter. Subsequent to his retirement, we entered into an agreement with Mr. Bihl on June 12, 2020, pursuant to which he received (i) a payment on June 16, 2020 of $9.25 million, which represented a $918,953 payment in full for amounts due to Mr. Bihl under the Phantom Plan, a $6,328,629 payment for the redemption of 150,252 of his Profits Interest Units under the MIP, and an additional cash payment of $2,006,796 to reflect the COVID-19-related decrease in value of his Phantom Plan award and the redeemed portion of his MIP award and (ii) a payment on February 8, 2021 of $12.3 million, which represented a $10,802,387 payment for the redemption of the remaining 183,078 of his Profits Interest Units under the MIP, and an additional cash payment of $1,543,147 to reflect the COVID-19-related decrease in value.
In connection with our IPO, we entered into new employment agreements with each of Messrs. Reali, Anglum, Nosenzo, D’Adamio, and Ms. Pavesio that became effective February 11, 2021 and superseded their previous severance arrangements. Under these new employment agreements, it is anticipated that, upon a termination without cause or resignation by the named executive officer with good reason, each of our named executive officers are entitled to (i) twelve months’ base salary (eighteen months in the case of Mr. Reali), payable in equal installments over the twelve month period (eighteen month period in the case of Mr. Reali) following such termination, (ii) 100% of target annual cash incentive (150% in the case of Mr. Reali), payable in equal installments over the twelve month period (eighteen month period in the case of Mr. Reali) following such termination, and (iii) payment of COBRA premiums for the first twelve months of coverage following termination of employment (eighteen months in the case of Mr. Reali). Additionally, upon a termination without cause or resignation by the named executive officer with good reason within the 24 month period following a change in control, each of our named executive officers are entitled to (i) eighteen months’ base salary (twenty-four months in the case of Mr. Reali), payable in a lump sum within 60 days following such termination, (ii) 150% of target annual cash incentive (200% in the case of Mr. Reali), payable in a lump sum within 60 days following such termination, (iii) a lump sum payment equal to eighteen months (twenty-four months in the case of Mr. Reali) of COBRA premiums within 60 days following such termination, and (iv) full vesting acceleration of all equity awards. These severance payments will be conditioned upon execution and delivery of a release and compliance with the restrictive covenants described below in “—Restrictive Covenants.”
Restrictive Covenants
As of December 31, 2020 our named executive officers were subject to certain post-employment restrictive covenants, including twelve-month non-competition and non-solicitation obligations, as set forth in proprietary information agreements entered into with each named executive officer. Further, the employment letters for each of our named executive officers provide for mutual non-disparagement obligations.
In connection with our IPO, we entered into new post-employment restrictive covenants with our named executive officers, effective as of the date of tour IPO, including twelve-month (and eighteen months in the case of Mr. Reali) noncompetition and non-solicitation obligations (increased to eighteen-months (and twenty-four months in the case of Mr. Reali) in the event a named executive officer receives change in control severance, as described above) and perpetual confidentiality and non-disparagement obligations.
Retirement Plans
BV LLC currently maintains a 401(k) retirement savings plan, or the 401(k) plan, in which all BV LLC employees, including our named executive officers, who satisfy certain eligibility requirements may participate. The Internal Revenue Code allows eligible employees to defer a portion of their compensation, within prescribed limits, on a pre-tax basis through contributions to the 401(k) plan. Under the terms of the 401(k) plan, we currently make (a) non-discretionary matching contributions equal to 50% of the employee’s contributions, up to a maximum of 6% of the employee’s eligible compensation and (b) a non-elective contribution equal to 4.5% of the employee’s compensation for the plan year. Due to the COVID-19 crisis, we suspended the 4.5% non-elective contribution effective May 3, 2020, but have reinstated such benefit effective December 26, 2020. Further, our board of managers has discretion under the 401(k) plan to provide for (i) annual discretionary matching contributions based on eligible compensation contributed by each employee and (ii) discretionary non-elective contributions in an amount determined by the board at year end, subject to continued employment through year end. We believe that providing a vehicle for tax-deferred retirement savings though the 401(k) plan adds to the overall desirability of our executive compensation package and further incentivizes our employees, including our named executive officers, in accordance with our compensation policies. We anticipate that our employees will continue to be eligible to participate in a 401(k) plan maintained by us.
Employee Benefits
All of our full-time employees and working partners, including our named executive officers, are eligible to participate in health and welfare plans maintained by BV LLC, including:
•medical, dental and vision benefits;
•medical flexible spending accounts and health savings account;
•short-term and long-term disability insurance;
•basic life and accidental death & dismemberment insurance; and
•group accident, critical illness and hospital indemnity plans.
Our named executive officers participate in these plans on the same basis as other eligible employees. We do not maintain any supplemental health and welfare plans for our named executive officers. We reimburse our named executive officers for the full cost of their personal cellular phones. We believe the benefits described above are necessary and appropriate to provide a competitive compensation package to our named executive officers.
Section 280G
The employment letters for Messrs. Reali, Anglum and D’Adamio in effect as of December 31, 2020 and the new employment agreements we entered into with our named executive officers in connection with our IPO provide that, in the case of their receipt of any payments in connection with a change in control (as defined in the employment letter or agreement), or that would otherwise be considered an “excess parachute payment” within the meaning of Section 280G of the Code, such payments will be reduced to the maximum amount that does not trigger the excise tax imposed by Section 4999 of the Code if the named executive officer would be better off on a net after-tax basis with such reduction.
Retention Plan
On April 13, 2020, we initiated a retention plan with Mr. Nosenzo for an aggregate amount of $520,000 less applicable taxes. Payments of $260,000 will be paid on each of May 4, 2021 and May 4, 2022, subject to Mr. Nosenzo’s continued service through each such date; provided that if Mr. Nosenzo’s employment is terminated for a reason that would qualify Mr. Nosenzo for severance benefits under his offer letter (x) before the May 4, 2021 payment date he will receive $260,000 in a single lump sum within 60 days following termination of employment and (y) after the May 4, 2021 payment date but before the May 4, 2022 payment date he will receive $260,000 in a lump sum within 60 days following the termination date. Any such payments are in addition to any severance benefits under Mr. Nosenzo’s offer letter.
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes the number of option awards and profits interest units (including the number of profits interest units underlying Phantom Units) for our named executive officers as of December 31, 2020. For additional information about the outstanding equity awards granted to our named executive officers, please see the section titled “—Equity-based compensation” above.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Option Awards(5) | Stock Awards |
| Name | | Grant Date | | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of profits interest units (including profits interest units underlying phantom units(1) that have not vested(2) (#) | | Market Value(3) of profits interest units (including profits interest units underlying phantom units) that have not vested ($) |
| Kenneth Reali | | 4/13/2020 | | | | | | 417,804 | (4) | $ | 7,716,840 | |
| | 7/30/2020 | | 5,935 | | — | $ | 42.12 | | 7/30/2021 | | | |
| Gregory O. Anglum | | 04/04/2016 | | | | | | 2,000 | (6) | 94,300 | |
| | 05/01/2017 | | | | | | 28,500 | (6) | 1,257,420 | |
| | 9/17/2018 | | | | | | 20,000 | (6) | 580,600 | |
| John E. Nosenzo | | 02/06/2017 | | | | | | 31,250 | (7) | 1,378,750 | |
| | 9/17/2018 | | | | | | 25,000 | (7) | 725,750 | |
| Anthony D’Adamio | | 8/14/2017 | | | | | | 14,000 | (8) | 617,680 | |
| | 9/17/2018 | | | | | | 15,000 | (8) | 435,450 | |
| Alessandra Pavesio | | 9/17/2018 | | | | | | 20,000 | (9) | 580,600 | |
___________
(1)The Phantom Units do not have an expiration date; provided that any Phantom Units granted on September 17, 2018 that do not vest as a result of achieving 2020 revenue targets on June 1, 2021 will expire.
(2)This column shows the number of profits interest units held by Mr. Bihl and the number of Phantom Units held by our other named executive officers that have not vested. Phantom Units generally represent the right to receive cash amounts from us based on the distributions that would be made to an equivalent number of profits interests with an equivalent benchmark amount. The benchmark amounts represent the cumulative distributions that must be made by us pursuant to the Bioventus LLC Agreement before a grantee is entitled to receive any distributions or payments in respect of such grantee’s units. The benchmark amount for Mr. Anglum’s 2016 grant of Phantom Units is $472,003,000, for Messrs. Nosenzo, Anglum, and D’Adamio’s 2017 grant is $510,000,000, for Messrs. Anglum, Nosenzo and D’Adamio’s and Ms. Pavesio’s 2018 grant of Phantom Units is $703,691,178, and for Mr. Reali’s 2020 grant of Phantom Units on June 25, 2020 is $840,849,878.
(3)Market value is determined based on an independent valuation report on the fair market value of the Company.
(4)Mr. Reali was granted 417,804 Phantom Units on June 25, 2020; 20% of such grant will vest on April 13, 2021 and 5% will vest each quarter thereafter.
(5)Mr. Reali was granted 5,935 options to purchase equity interests of BV LLC on July 30, 2020, all of which were fully vested and exercisable at the time of grant.
(6)Mr. Anglum was granted 20,000 Phantom Units on April 4, 2016 and 95,000 Phantom Units on May 1, 2017; 20% of each grant vested on the first anniversary of the grant date and 5% vests each quarter thereafter. Mr. Anglum was also granted 20,000 Phantom Units on September 17, 2018; these units will vest on June 1, 2021 at 100% if 2020 revenue is greater than or equal to $391.8 million and at 75% if 2020 revenue is greater than or equal to $336.0 million.
(7)Mr. Nosenzo was granted 125,000 Phantom Units on February 6, 2017; 20% of these Phantom Units vested on February 6, 2018 and 5% vests each quarter thereafter. Mr. Nosenzo was also granted 25,000 Phantom Units on September 17, 2018; these units will vest on June 1, 2021 at 100% if 2020 revenue is greater than or equal to $391.8 million and at 75% if 2020 revenue is greater than or equal to $336.0 million.
(8)Mr. D’Adamio was granted 40,000 Phantom Units on August 14, 2017; 20% of such grant vested on the first anniversary of the grant date and 5% vests each quarter thereafter. Mr. D’Adamio was also granted 15,000 Phantom Units on September 17, 2018; these units will vest on June 1, 2021 at 100% if 2020 revenue is greater than or equal to $391.8 million and at 75% if 2020 revenue is greater than or equal to $336.0 million.
(9)Ms. Pavesio was granted 20,000 Phantom Units on September 17, 2018; these units will vest on June 1, 2021 at 100% if 2020 revenue is greater than or equal to $391.8 million and at 75% if 2020 revenue is greater than or equal to $336.0 million. Ms. Pavesio was also granted 83,333 Phantom Units on July 22, 2013, 15,000 Phantom Units on June 1, 2015, and 11,392 Phantom Units on April 21, 2016, all of which were fully vested as of December 31, 2020.
Director Compensation
The following table sets forth information concerning the compensation of the current members of the BV LLC board of managers for the year ended December 31, 2020.
| | | | | | | | | | | | | | | | | | | | |
| Name | | Year | | Fees Earned or Paid in Cash ($)(1) | | Total ($)(2) |
| William A. Hawkins(3) | | 2020 | | $ | 90,000 | | | $ | 90,000 | |
| Susan M. Stalnecker(3) | | 2020 | | 60,000 | | | 60,000 | |
| Guy P. Nohra | | 2020 | | — | | | — | |
| Martin P. Sutter | | 2020 | | — | | | — | |
| Bradley J. Cannon | | 2020 | | — | | | — | |
| David J. Parker | | 2020 | | — | | | — | |
| Philip G. Cowdy | | 2020 | | — | | | — | |
| Guido J. Neels | | 2020 | | — | | | — | |
___________
(1)Mr. Hawkins received an annual retainer of $40,000 for his service as a member of the board and an additional annual retainer fee of $50,000 for his service as chairman of the board. Ms. Stalnecker received an annual retainer fee of $50,000 for her service as a member of the board and an additional annual retainer fee of $10,000 for her service on the Audit and Risk Committee of the board. No other members of our board received any cash compensation in 2020.
(2)No members of our board received equity compensation awards in 2020.
(3)As of December 31, 2020, Mr. Hawkins held 50,000 Phantom Units, 95% of which were vested and 5% of which were unvested, and Ms. Stalnecker held 50,000 Phantom Units, 40% of which were vested and 60% of which were unvested, respectively. The benchmark amount for Mr. Hawkins’s grant of Phantom Units is $472,003,000 and the benchmark amount for Ms. Stalnecker’s grant of Phantom Units is $703,691,178.
In connection with our December 11, 2015 offer to Mr. Hawkins to join the BV LLC board of managers as its chairman, we agreed, pursuant to an offer letter, effective January 1, 2016, to (1) pay Mr. Hawkins an annual retainer fee of $40,000 for his service as a member of the board and $50,000 for his service as chairman of the board, each payable in quarterly installments in arrears and pro-rated for any partial period of service and (2) award Mr. Hawkins a one-time grant of 50,000 Phantom Units under the Phantom Plan.
Effective November 28, 2018, pursuant to an offer letter with Ms. Stalnecker providing for her appointment as a member of our board and chair of the Audit and Risk Committee, we agreed to (1) pay Ms. Stalnecker an annual retainer fee of $50,000 for her service as a member of the board and $10,000 for her participation in the Audit and Risk Committee, each payable in quarterly installments in arrears and pro-rated for any partial period of service and (2) award Ms. Stalnecker 50,000 Phantom Units under the Phantom Plan. Ms. Stalnecker received a prorated payment of $9,167 for her time on the board and on the Audit and Risk Committee in 2018.
Our non-employee director compensation policy, which became effective February 11, 2021, provides that each non-employee director receives an annual cash retainer of $55,000. In addition, (i) the Chairperson of the Board shall receive an additional annual retainer of $50,000, (ii) the Lead Director of the Board shall receive an additional annual retainer of $30,000, (iii) the Chairpersons of the Audit and Risk Committee, Compensation Committee, Nominating and Corporate Governance Committee, and Compliance, Ethics and Culture Committee shall receive additional annual retainers of $20,000, $15,000, $10,000 and $10,000, respectively, and (iv) non-Chairperson members of the Audit and Risk Committee, Compensation Committee, Nominating and Corporate Governance Committee, and Compliance, Ethics and Culture Committee shall receive additional annual retainers of $10,000, $7,500, $5,000 and $5,000, respectively. In addition, each such non-employee director receives an annual RSU award with a grant date value of $152,000, with all such annual RSU awards (other than those received in respect of a non-employee director’s initial year of service, as described below) vesting on the first anniversary of the grant date of the award (or immediately prior to the date of the annual shareholder meeting immediately following the date of grant, if sooner), subject to such non-employee director continuing in service through such date. RSU awards for each non-employee director’s initial year of service shall vest in three equal installments, with the first installment vesting on the first anniversary of the grant date of such initial award (or immediately prior to the date of the annual shareholder meeting immediately following the date of grant, if sooner) and the second and third installments vesting on the first and second anniversaries of such first vesting date, subject to such non-employee director continuing in service through each such date (and any such nonemployee director who commences service on a date other than the date of the annual shareholder meeting receives a pro-rata restricted stock unit award for such initial year of service). In addition, on February 11, 2021, in connection with our IPO, each non-employee director received a RSU award with a grant date value of $152,000 that vests on the first anniversary of the grant date of the award. Each RSU award awarded under the policy accelerates and vests in full upon a change in control (as defined in the 2021 Incentive Plan). In addition, each non-employee director is reimbursed for out-of-pocket expenses in connection with his or her services.
Item 12.Security Ownership of Certain Beneficial Owner and Management and Related Stockholder Matters.
Securities authorized for issuance under equity compensation plans
As of December 31, 2020, there was no public market for our common stockThe information required by this Item is incorporated by reference from the section captioned “Equity Compensation Plan Information” and there were no equity compensation plans approved by security holders under which our equity securities were authorized for issuance. The following table provides, as of March 22, 2021, equity compensation plan information for all plans under which equity securities are authorized for issuance.
| | | | | | | | | | | | | | | | | | | | |
| | Number of securities to be issued upon exercise of outstanding options and rights
(a) | | Weighted-average exercise price of outstanding options and rights
(b) | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected under column (a))(4) (c) |
Equity compensation plans approved by security holders(1) | | 4,922,170 | | (2) | $ | 13.00 | | (3) | 3,212,626 | |
| Equity compensation plans not approved by security holders | | — | | | — | | | — | |
(1)Consists of the Bioventus Inc. 2021 Incentive Award Plan (2021 Plan) and the Bioventus Inc. 2021 Employee Stock Purchase Plan (2021 ESPP).
(2)Includes 4,561,500 outstanding options to purchase shares of our Class A common stock and 360,670 outstanding restricted stock units under the 2021 Plan. There were no agreements outstanding to purchase shares of our Class A common stock under the 2021 ESPP.
(3)The weighted average exercise price in column (b) does not take into account outstanding restricted stock units, which do not have an exercise price.
(4)Includes 2,670,306 shares of Class A common stock available for future issuance under the 2021 Plan and 542,320 shares of Class A common stock available for future issuance under the 2021 ESPP. The number of shares of Class A common stock reserved for issuance under the 2021 Plan will automatically increase on January 1 of each calendar year from January 1, 2022 through January 1, 2031, by that number of shares of Class A common stock equal to the lesser of (i) 4.5% of the shares of all of the classes of our Class A common stock outstanding on the last day of the immediately preceding fiscal year and (ii) such smaller number of shares of Class A common stock as determined by the board of directors. The number of shares of Class A common stock reserved for issuance under the 2021 ESPP will automatically increase on January 1 of each calendar year from January 1, 2022 through January 1, 2031, by that number of shares of Class A common stock equal to the lesser of (A) 1% of the shares of all of our Class A common stock outstanding on the final day of the immediately preceding calendar year and (B) such smaller number of shares of Class A common stock as determined by the board of directors.
Prior to our IPO, BV LLC maintained the Phantom Plan, pursuant to which we granted Units in order to foster and promote our long-term success by helping attract and maintain a superior management team and to motivate superior performance by employees selected to participate in the Phantom Plan. As of December 31, 2020, there were 1,651,709 units outstanding. In connection with our IPO, we terminated the Phantom Plan and Bioventus Inc. assumed the obligations of BV LLC. Vested Phantom Plan awards granted to employees that were active employees on February 11, 2021 will be paid in the form of shares of Class A common stock after the one year anniversary. The number of shares of Class A common stock were determined by dividing (i) the value of the participant’s vested Phantom Units (after giving effect to any accelerations in vesting in connection with our IPO) as of February 11, 2021 by (ii) the $13.00 IPO price of our Class A common stock. Upon the one year anniversary of our IPO 798,422 shares of Class A common stock will be issued in settlement of the outstanding units.
Security“Security Ownership of Certain Beneficial OwnerOwners and Management
The following table presents information asManagement” contained in our definitive proxy statement for our 2024 Annual Meeting of Stockholders to the beneficial ownership of our Class A common stock and Class B common stock, and of March 22, 2021 for:
•each person, or group of affiliated persons, known by us to beneficially own more than 5% of our Class A common stock or our Class B common stock;
•each named executive officer;
•each of our directors; and
•all executive officers and directors as a group.
As described in Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Bioventus LLC Agreement, the Continuing LLC Owner is entitled to have its LLC Interests (as defined below) redeemed for Class A common stock on a one-for-one basis, or, if we and the Continuing LLC Owner agree, a cash payment equal to the market value of the applicable number of our shares of Class A common stock. In addition, at Bioventus’ election, Bioventus may effect a direct exchange of such Class A common stock or such cash (if mutually agreed) for such LLC Interests in lieu of such a redemption. In connection with our IPO, we issued to the Continuing LLC Owner one share of Class B common stock for each LLC Interest it owns. As a result, the number of shares of Class B common stock listed in the table below correlates to the number of LLC Interests the Continuing LLC Owner owns.
The number of shares beneficially owned by each stockholder is determined under rules issued by the SEC and includes voting or investment power with respect to securities. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of common stock subject to options, or other rights, including the redemption right described above, held by such person that are currently exercisable or will become exercisable within 60 days of March 22, 2021, are considered outstanding, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person. Unless otherwise indicated, the address of each of the individuals and entities named below is c/o Bioventus Inc., 4721 Emperor Boulevard, Suite 100, Durham, NC 27703. Each of the stockholders listed has sole voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community property laws where applicable.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Shares of Class A Common Stock Beneficially Owned | | Shares of Class B Common Stock Beneficially Owned | | Combined Voting Power(1) |
| Name of Beneficial Owner | | Number | | % | | Number | | % | | % |
| 5% Stockholders | | | | | | | | | | |
EW Healthcare Partners (2) | | 13,021,324 | | | 31.7 | % | | — | | | * | | 22.9 | % |
Smith & Nephew (3) | | 6,229,991 | | | 15.2 | % | | 15,786,737 | | | 100 | % | | 38.7 | % |
Spindletop Healthcare Capital L.P.(4) | | 3,906,395 | | | 9.5 | % | | — | | | * | | 6.9 | % |
Ampersand Capital(5) | | 3,255,332 | | | 7.9 | % | | — | | | * | | 5.7 | % |
Pantheon Global Co-Investment Opportunities Fund L.P.(6) | | 2,821,283 | | | 6.9 | % | | — | | | * | | 5.0 | % |
Alta Partners VIII, L.P.(7) | | 2,604,264 | | | 6.3 | % | | — | | | * | | 4.6 | % |
Integrated Core Strategies (US) LLC(8) | | 2,432,581 | | | 5.9 | % | | — | | | * | | 4.3 | % |
| Name Executive Officers and Directors | | | | | | | | | | |
| Kenneth M. Reali | | 9,375 | | | * | | — | | | * | | * |
| Gregory O. Anglum | | 6,000 | | | * | | — | | | * | | * |
| John E. Nosenzo | | 500 | | | * | | — | | | * | | * |
| Anthony D’Adamio | | 1,000 | | | * | | — | | | * | | * |
| Alessandra Pavesio | | — | | | * | | — | | | * | | * |
| William A. Hawkins | | — | | | * | | — | | | * | | * |
| Philip G. Cowdy | | — | | | * | | — | | | * | | * |
| Guido J. Neels | | — | | | * | | — | | | * | | * |
| Guy P. Nohra | | — | | | * | | — | | | * | | * |
| David J. Parker | | — | | | * | | — | | | * | | * |
| Martin P. Sutter | | — | | | * | | — | | | * | | * |
| Susan M. Stalnecker | | — | | | * | | — | | | * | | * |
| All directors and executive officers as a group (13 persons) | | 16,875 | | | * | | — | | | * | | * |
____________________
*Represents beneficial ownership of less than 1%.
(1)Represents the voting power of each owner based on the voting power held through both the owner’s Class A common stock and Class B common stock. Represents percentage of voting power of the Class A common stock and Class B common stock of Bioventus voting together as a single class.
(2)Represents 12,096,702 shares held by EW Healthcare Partners Acquisition Fund, L.P., which we refer to as the “Essex Stockholder,” and 924,622 shares held by White Pine Medical, LLC, a subsidiary of the Essex Stockholder, which we refer to as “White Pine.” EW Healthcare Partners Acquisition Fund GP, L.P., a Delaware limited partnership, is the general partner of the Essex Stockholder and is referred to as the “Partnership,” and EW Healthcare Partners Acquisition Fund UGP, LLC, a Delaware limited liability company, is the general partner of the Partnership and is referred to as the “General Partner.” Martin P. Sutter, Petri Vainio, Ronald W. Eastman, and Scott Barry are the managers of the General Partner, and each is referred to as a “Manager” and collectively as the “Managers.” The Partnership is deemed to have sole voting and dispositive power with respect to the shares held by the Essex Stockholder and White Pine. The Managers are deemed to have shared voting and dispositive power with respect to the shares held by the Essex Stockholder and White Pine by unanimous consent and through the Partnership. Each Manager disclaims beneficial ownership of such shares except to the extent of his pecuniary interest therein. The address of the Essex Stockholder and White Pine is 21 Waterway Avenue, Suite 225, The Woodlands, Texas 77380.
(3)Represents the shares of Class A common stock held by Smith & Nephew (Europe) B.V., a wholly owned indirect Dutch subsidiary of Smith & Nephew plc, and the shares of Class B common stock held by Smith & Nephew, Inc., a wholly owned indirect U.S. subsidiary of Smith & Nephew plc. The address of Smith & Nephew (Europe) B.V. is Bloemlaan 2, 2132 NP Hoofddorp, Netherlands. The address of Smith & Nephew, Inc. is 7135 Goodlett Farms, Cordova, Tennessee 38106.
(4)Represents shares held by Spindletop Healthcare Capital L.P. Evan Melrose, MD is the Manager of the General Partner of the General Partner of Spindletop Healthcare Capital L.P. and may be deemed to have shared voting and dispositive power with respect to the shares held by Spindletop Healthcare Capital L.P. The address of Spindletop Healthcare Capital L.P. is 3571 Far West Blvd., PMB #108, Austin, Texas 78731.
(5)Represents shares held by AMP-CF Holdings, LLC, which we refer to as the “Ampersand Capital Stockholder.” Herbert H. Hooper, the Managing Member of the General Partner of the General Partner of each of the members and managers of the Ampersand Capital Stockholder, may be deemed to have voting and dispositive power with respect to shares held by the Ampersand Capital Stockholder. The address of the Ampersand Capital Stockholder is in care of Ampersand Capital Partners, 55 William Street, Suite 240, Wellesley, Massachusetts 02481.
(6)Represents shares held by Pantheon Global Co-Investment Opportunities Fund L.P. David Braman, Susan Long McAndrews and Lily Wong are directors of Pantheon Global Co-Investment Opportunities GP Limited, the general partner of Pantheon Global Co-Investment Opportunities Fund, L.P. and make the investment and voting decisions with respect to shares held by of Pantheon Global Co-Investment Opportunities Fund, L.P. The address of Pantheon Global Co-Investment Opportunities Fund L.P. is 600 Montgomery Street, 23rd Floor, San Francisco, CA 94111.
(7)Represents shares held by Alta Partners VIII, L.P. Alta Partners Management VIII, LLC is the general partner of Alta Partners VIII, L.P. Guy Nohra, Daniel Janney and Farah Champsi are managing directors of Alta Partners Management VIII, LLC and exercise shared voting and investment powers with respect to the shares owned by Alta Partners VIII, L.P. Each of the reporting persons disclaims beneficial ownership of such shares, except to the extent of their proportionate pecuniary interest therein, if any. The principal business address of Alta Partners VIII, L.P. is One Embarcadero Center, 37th Floor San Francisco, CA 94111.
(8)Pursuant to the Schedule 13G filed with the SEC by Integrated Core Strategies (US), LLC (Integrated Core Strategies) et al, reporting ownership of these shares of Class A common stock as of March 16, 2021. As reported in said Schedule 13G, Integrated Core Strategies reports shared voting and dispositive power with respect to 2,033,399 shares of Class A common stock, ICS Opportunities II LLC (ICS Opportunities II) reports shared voting and dispositive power with respect to 75,287 shares of Class A common stock, ICS Opportunities, Ltd. (ICS Opportunities) reports shared voting and dispositive power with respect to 310,116 shares of Class A common stock, Integrated Assets, Ltd. (Integrated Assets) reports shared voting and dispositive power with respect to 13,779 shares of Class A common stock, Millennium International Management LP (Millennium International Management) reports shared voting and dispositive power with respect to 399,182 shares of Class A common stock, Millennium Management LLC (Millennium Management) reports shared voting and dispositive power with respect to 2,432,581 shares of Class A common stock, Millennium Group Management LLC (Millennium Group Management) reports shared voting and dispositive power with respect to 2,432,581 shares of Class A common stock, and Israel A. Englander reports shared voting and dispositive power with respect to 2,432,581 shares of Class A common stock. Millennium International Management iswithin 120 days after the investment manager to ICS Opportunities II, ICS Opportunities and Integrated Assets (collective, the ICS Entities) and is deemed to have beneficial ownership of their respective securities. Millennium Management is the general partnerend of the managing member of Integrated Core Strategies and the general partner of the 100% owner of the ICS Funds is deemed to have beneficial ownership of the securities owned by each of Integrated Core Strategies and the ICS Entities (collectively, the Integrated Entities). Millennium Group Management is the managing member of Millennium Management and general partner of Millennium International Management and is deemed to have beneficial ownership of the securities owned by the Integrated Entities. The managing member of Millennium Group Management is a trust of which Mr. Englander, a United States citizen, currently serves as the sole voting trustee, and therefor Mr. Englander is deemed to have beneficial ownership of securities owned by the Integrated Entities. The address of Mr. Englander and each of the foregoing entities is 399 Park Avenue, New York, New York 10022.fiscal year ended December 31, 2023.
Item 13.Certain Relationships and Related Transactions, and Director IndependenceIndependence.
PoliciesThe information required by this Item is incorporated by reference from the section captioned “Certain Relationships and Procedures for Related Person Transactions
Our Board has adopted a written Related Party Transaction Policy, which sets forth the policies and proceduresTransactions” contained in our definitive proxy statement for the review and approval or ratificationour 2024 Annual Meeting of related person transactions. This policy covers, with certain exceptions set forth in Item 404 of Regulation S-K, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were (or any of our subsidiaries) areStockholders to be a participant, where the amount involved exceeds $120,000 since the beginning of the prior fiscal year and a related person had, has or will have a direct or indirect material interest.
Under the policy, our legal team is primarily responsible for developing and implementing procedures to obtain information regarding related persons andfiled with respect to potential related person transactions, and then determining, based on the facts and circumstances, whether such transactions constitute related person transactions subject to the policy. Our general counsel is then required to present to the Audit and Risk Committee each proposed related person transaction. In reviewing and approving any such transactions, our Audit and Risk Committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction.
If advance Audit and Risk Committee approval of a related person transaction is not feasible, then the transaction may be preliminarily entered into by management upon prior approval by the chairperson of the Audit and Risk Committee, subject to ratification of the transaction by the Audit and Risk Committee at such committee’s next regularly scheduled meeting. Management is responsible for updating the Audit and Risk Committee as to any material changes to any approved or ratified related person transaction and for providing a status report at least annually of all then current related person transactions at a regularly scheduled meeting of the Audit and Risk Committee. No director may participate in approval of a related person transaction for which he or she is a related person. Unless noted otherwise, all of the transactions, agreements or relationships described in this section occurred prior to the adoption of this policy.
Since January 1, 2020, we engaged in certain transactions for which the amount involved exceeds $120,000 with our directors, executive officers or beneficial holders of more than 5% of our Class A common stock, or immediate family member thereof. The following are summaries of certain provisions of our agreements with related persons and are qualified in their entirety by reference to all of the provisions of such agreements. Because these descriptions are only summaries of the applicable agreements, they do not necessarily contain all of the information that you may find useful. We therefore urge you to review the agreements in their entirety. Copies of the agreements (or forms of the agreements) are included as exhibits to this Annual Report on Form 10-K, and are available electronically on the website of the SEC at www.sec.gov.
Our IPO and Transactions
In connection with the IPO, we engaged in certain transactions with certain of our directors, executive officers and other persons and entities which are or became holders of 5% or more of our voting securities upon the consummation of the IPO and including, without limitation (a) the amendment and restatement of the limited liability company agreement of BV LLC (Bioventus LLC Agreement) entered into by us and Smith & Nephew Inc. (the Continuing LLC Owner) to, among other things, (i) provide for a new single class of common membership interests in BV LLC (LLC Interests), (ii) exchange all of the then existing membership interests of the holders of BV LLC membership interests (Original LLC Owners) for LLC Interests and (iii) appoint Bioventus Inc. as the sole managing member of BV LLC; (b) the acquisition, by various mergers, certain members of BV LLC prior to the IPO (the Former LLC Owners) for which we issued 31,838,589 shares of Class A common stock as merger consideration (Merger); (c) entry into a tax receivable agreement (TRA) with the Continuing LLC Owner, the Stockholders Agreement (as defined below) and the Registration Rights Agreement (as defined below) and (d) the satisfaction of a $3.3 million cash entitlement of the Continuing LLC Owner, in respect of an equity participation right unit held by the Continuing LLC Owner.
The Merger involved the exchange of the indirect ownership interest in LLC Interests held by Former LLC Owners and occurred as a result of various mergers, whereby, in each case, a newly formed subsidiary of Bioventus merged into an entity that holds LLC Interests (and of which the Former LLC Owners were owners) with each entity becoming a wholly owned subsidiary of Bioventus and the Former LLC Owners receiving Class A common stock. Each Former LLC Owner and/or one or more its affiliates has agreed, pursuant to their respective merger agreement, to indemnify us against all historic liabilities of the entity transferred in the applicable merger. Subsequent to these mergers, each such entity merged into Bioventus resulting in Bioventus owning directly the LLC Interests exchanged by the Former LLC Owners pursuant to the initial mergers. In connection with the Merger, the aggregate fair value of the Class A common stock transferred to certain of the Former LLC Owners, including EW Healthcare Partners, Smith & Nephew (Europe) B.V., Spindletop Healthcare Capital L.P., Ampersand Capital, Pantheon Global Co-Investment Opportunities Fund L.P. and Alta Partners VIII, L.P., each beneficially owing 5% or more of our Class A common stock, was approximately $413.9 million.
Tax Receivable Agreement
We expect to obtain an increase in our share of the tax basis of the assets of BV LLC when the Continuing LLC Owner receives shares of our Class A common stock or, if we and the Continuing LLC Owner agree, cash in connection with an exercise of the Continuing LLC Owner’s right to have its LLC Interests redeemed by BV LLC or, at our election, directly exchanged (such basis increases, together with the basis increases resulting from certain distributions (or deemed distributions) from BV LLC, the “Basis Adjustments”). We intend to treat such redemptions or exchanges of LLC Interests as the direct purchase of LLC Interests by us from the Continuing LLC Owner for U.S. federal income and other applicable tax purposes, regardless of whether such LLC Interests are surrendered by the Continuing LLC Owner to BV LLC for redemption or sold to us upon the exercise of our election to acquire such LLC Interests directly. A Basis Adjustment may have the effect of reducing the amounts that we would otherwise pay in the future to various tax authorities. The Basis Adjustments may also decrease gains (or increase losses) on future dispositions of certain capital assets to the extent tax basis is allocated to those capital assets.
We entered into the TRA with the Continuing LLC Owner. The TRA provides for our payment to the Continuing LLC Owner of 85% of the amount of tax benefits, if any, that we actually realize, or in some circumstances are deemed to realize, as a result of any Basis Adjustments and certain other tax benefits arising from payments under the TRA. BV LLC has in effect an election under Section 754 of the Code effective for each taxable year in which a redemption or exchange (including deemed exchange) of LLC Interests for shares of our Class A common stock or cash occurs. These TRA payments are not conditioned upon any continued ownership interest in either BV LLC or us by the Continuing LLC Owner. The rights of the Continuing LLC Owner under the TRA are assignable to transferees of its LLC Interests (other than us as transferee pursuant to subsequent redemptions or exchanges of the transferred LLC Interests). We expect to benefit from the remaining 15% of tax benefits, if any, that we may actually realize.
The actual Basis Adjustments, as well as any amounts paid to the Continuing LLC Owner under the TRA, will vary depending on a number of factors, including:
•the timing of any subsequent redemptions or exchanges—for instance, the increase in any tax deductions will vary depending on the fair value, which may fluctuate over time, of the depreciable or amortizable assets of BV LLC at the time of each redemption or exchange;
•the price of shares of our Class A common stock at the time of redemptions or exchanges—the Basis Adjustments, as well as any related increase in any tax deductions, is directly related to the price of shares of our Class A common stock at the time of each redemption or exchange;
•the extent to which such redemptions or exchanges are taxable—if a redemption or exchange is not taxable for any reason, increased tax deductions will not be available; and
•the amount and timing of our income—the TRA generally requires us to pay 85% of the tax benefits as and when those benefits are treated as realized under the terms of the TRA. Except as discussed below in cases of (i) a material breach of a material obligation under the TRA, (ii) a change of control or (iii) an early termination of the TRA, if we do not have taxable income, it will generally not be required to make payments under the TRA for that taxable year because no tax benefits will have been realized. However, any tax benefits that do not result in realized tax benefits in a given taxable year may generate tax attributes that may be utilized to generate tax benefits in future taxable years. The utilization of any such tax attributes will result in payments under the TRA.
For purposes of the TRA, cash savings in income tax will be computed by comparing our actual income tax liability to the amount of such taxes that it would have been required to pay had there been no Basis Adjustments and had the TRA not been entered into. The TRA generally applies to each of our taxable years, beginning with the first taxable year endingwithin 120 days after the consummationend of the offering. There is no maximum term for the TRA; however, the TRA may be terminated by us pursuant to an early termination procedure that requires us to pay the Continuing LLC Owner an agreed upon amount equal to the estimated present value of the remaining payments to be made under the agreement (calculated based on certain assumptions, including regarding tax rates and utilization of the Basis Adjustments).
The payment obligations under the TRA are our obligation and not BV LLC’s. Although the actual timing and amount of any payments that may be made under the TRA will vary, we expect that the payments that we may be required to make to the Continuing LLC Owner could be significant. Any payments made by us to the Continuing LLC Owner under the TRA generally reduces the amount of overall cash flow that might have otherwise been available to us or to BV LLC and, to the extent that we are unable to make payments under the TRA for any reason, the unpaid amounts generally are deferred and accrue interest until paid by us.
Decisions made by us in the course of running our business, such as with respect to mergers, asset sales, other forms of business combinations or other changes in control, may influence the timing and amount of payments that are received by the Continuing LLC Owner under the TRA. For example, the earlier disposition of assets following a transaction that results in a Basis Adjustment generally accelerate payments under the TRA and increase the present value of such payments.
The TRA provides that if (i) we materially breach any of our material obligations under the TRA, (ii) certain mergers, asset sales, other forms of business combination, or other changes of control were to occur, on or before December 31, 2021 or (iii) we elect an early termination of the TRA, then our obligations, or our successor’s obligations, under the TRA would be based on certain assumptions, including an assumption that we would have sufficient taxable income to fully utilize all potential future tax benefits that are subject to the TRA. The TRA also provides that in the case of certain mergers, asset sales, other forms of business combination or other changes of control occurring on or after December 31, 2021, payments under the TRA would be based on certain assumptions, including an assumption that in each taxable year ending on or after the change of control, we would have taxable income equal to the greater of (A) actual taxable income for such taxable year and (B) the product of (x) four and (y) the highest taxable income in any of the four fiscal quarters ended prior to the change in control (increased by 10% for each taxable year beginning with the second taxable year following the change in control), in each case, as adjusted to take into account our actual percentage ownership in BV LLC for the taxable year for which the tax benefit payment is being determined.
As a result of the foregoing, (i) we could be required to make cash payments to the Continuing LLC Owner that are greater than the specified percentage of the actual benefits we ultimately realize in respect of the tax benefits that are subject to the TRA, and (ii) if we materially breach any of our material obligations under the TRA or if we elected to terminate the TRA early, we would be required to make an immediate cash payment equal to the present value of the anticipated future tax benefits that are the subject of the TRA, which payment may be made significantly in advance of the actual realization, if any, of such future tax benefits. In these situations, our obligations under the TRA could have a material adverse effect on our liquidity and could have the effect of delaying, deferring or preventing certain mergers, asset sales, other forms of business combination, or other changes of control. There can be no assurance that we will be able to finance our obligations under the TRA. We anticipate funding ordinary course payments under the TRA from distributions from BV LLC out of distributable cash, to the extent permitted by our agreements governing our indebtedness.
Payments under the TRA are based on the tax reporting positions that we determine. Pursuant to the TRA, the Continuing LLC Member is required to reimburse us for cash payments previously made to it pursuant to the TRA if any tax benefits actually realized by us are subsequently challenged by a taxing authority and ultimately disallowed. In addition, but without duplication of any amounts previously reimbursed by the Continuing LLC Owner, any excess cash payments made by us to the Continuing LLC Owner will be netted against any future cash payments that we might otherwise be required to make under the terms of the TRA. However, a challenge to any tax benefits actually realized by us may not arise for a number of years following the initial time of such payment and we might not determine that we have effectively made an excess cash payment to the Continuing LLC Owner for a number of years following the initial time of such payment. Moreover, there can be no assurance that any excess cash payments for which the Continuing LLC Owner has a reimbursement obligation under the TRA will be repaid to us. As a result, it is possible that we could make cash payments under the TRA that are substantially greater than our actual cash tax savings. The applicable U.S. federal income tax rules are complex and factual in nature, and we cannot assure you that the IRS or a court will not disagree with our tax reporting positions. We have full responsibility for, and sole discretion over, all of our and BV LLC’s tax matters, including the filing and amendment of all tax returns and claims for refund and defense of all tax contests, subject to certain participation and approval rights held by the Continuing LLC Owner.
Payments are generally due under the TRA within a specified period of time following the filing of our tax return for the taxable year with respect to which the payment obligation arises, although interest on such payments begin to accrue at a rate of LIBOR plus 100 basis points from the due date, without extensions, of such tax return and ending on the date that such payments are required to be made under the terms of the TRA. Any late payments that may be made under the TRA continue to accrue interest at LIBOR plus 500 basis points from the due date of such payments under the TRA until such payments are made, including any late payments that we may subsequently make because we did not have enough available cash to satisfy our payment obligations at the time at which they originally arose, including as a result of restrictions on payments to our equity owners in the agreements governing our indebtedness. There were no payments to the Continuing LLC Owner made by the Company pursuant to this agreement since January 1, 2020.
Bioventus LLC Agreement
We operate our business through BV LLC and its subsidiaries. We and the Continuing LLC Owner entered into the Bioventus LLC Agreement, which sets forth terms for the operations of BV LLC, and the rights and obligations of the holders of LLC Interests.
Appointment as Manager
Under the Bioventus LLC Agreement, we are a member and the sole manager of BV LLC. As the sole manager, we are able to control all of the day-to-day business affairs and decision-making of BV LLC. As such, we, through our officers and directors, are responsible for all operational and administrative decisions of BV LLC and the day-to-day management of BV LLC’s business. Pursuant to the terms of the Bioventus LLC Agreement, we cannot, under any circumstances, be removed as the sole manager of BV LLC except by our election.
Compensation
We are not entitled to compensation for our services as manager. We are entitled to reimbursement or capital contribution credit by BV LLC for fees and expenses incurred on behalf of BV LLC, including all expenses associated with our IPO and maintaining our corporate existence.
Recapitalization.
The Bioventus LLC Agreement recapitalizes the units that were held prior to the IPO in BV LLC into a new single class of common membership units, which we refer to as the “LLC Interests.” Each LLC Interest entitles the holder to a pro rata share of the net profits and net losses and distributions of BV LLC.
Distributions
The Bioventus LLC Agreement requires “tax distributions” to be made by BV LLC to its members, as that term is defined in the agreement. Tax distributions will be made to members on a pro rata basis, including us, in an amount sufficient to allow the members, including us, to pay taxes owed in respect of income allocated by BV LLC and to allow us to meet our obligations under the TRA (as described above under the heading “Tax Receivable Agreement” above). For tax distributions made in the fiscal year endingended December 31, 2021 (the 2021 fiscal year), the tax rate that we expect to use for purposes of determining tax distributions from BV LLC to its members will equal the combined federal, state, and local statutory tax rate applicable to us for the 2021 fiscal year, taking into account the deductibility of state and local taxes for federal purposes. For each subsequent fiscal year, the tax rate applicable to us for the 2021 fiscal year will apply with respect to tax distributions made during such fiscal year unless our Board determines otherwise. The tax rate used to determine tax distributions will apply regardless of the actual final tax liability of any such member. Tax distributions will also be made only to the extent all distributions from BV LLC for the relevant period were otherwise insufficient to enable each member to cover its tax liabilities as calculated in the manner described above. The Bioventus LLC Agreement allows for distributions to be made by BV LLC to its members on a pro rata basis out of “distributable cash,” as that term is defined in the agreement. We expect BV LLC may make distributions out of distributable cash periodically to the extent permitted by our agreements governing our indebtedness and necessary to enable us to cover our operating expenses and other obligations, including our tax liability and obligations under the TRA, as well as to make dividend payments, if any, to the holders of our Class A common stock.
LLC Interest Redemption Right
The Bioventus LLC Agreement provides a redemption right to the Continuing LLC Owner which entitles it to have its LLC Interests redeemed, at its election, for newly-issued shares of our Class A common stock on a one-for-one basis or a cash payment equal to a volume weighted average market price of one share of Class A common stock for each LLC Interest redeemed (subject to customary adjustments, including for stock splits, stock dividends and reclassifications). If the Continuing LLC Owner elects to receive a cash payment, we may elect to settle such redemption with Class A common stock in lieu of a cash payment, provided that if we elect to do so, the Continuing LLC Owner has the option to rescind its redemption request within a specified time period. Upon the exercise of the redemption right, the redeeming member will surrender its LLC Interests to BV LLC for cancellation. The Bioventus LLC Agreement requires that we contribute cash or shares of our Class A common stock to BV LLC in exchange for an amount of newly-issued LLC Interests in BV LLC that will be issued to us equal to the number of LLC Interests redeemed from the Continuing LLC Owner. BV LLC will then distribute the cash or shares of our Class A common stock to the Continuing LLC Owner to complete the redemption. In the event of such a redemption election by the Continuing LLC Owner, we may effect a direct exchange of cash or Class A common stock for such LLC Interests in lieu of such a redemption. Whether by redemption or exchange, we are obligated to ensure that at all times the number of LLC Interests that we own equals the number of shares of Class A common stock issued by us (subject to certain exceptions for treasury shares and shares underlying certain convertible or exchangeable securities).
Indemnification
The Bioventus LLC Agreement provides for indemnification of the manager, members and officers of BV LLC and their respective subsidiaries or affiliates.
Stockholders Agreement
The Former LLC Owners and Continuing Owner (Voting Group), which hold Class A common stock or Class B common stock representing approximately 83.8% of the combined voting power of our Class A and Class B common stock as of March 22, 2021, entered into a stockholders agreement (Stockholders Agreement) with us on February 16, 2021.
Voting Agreement
Pursuant to the terms of the Stockholders Agreement, EW Healthcare Partners, Spindletop Healthcare Capital, Pantheon Global, Ampersand Capital, Alta Partners and their respective affiliates, as members of the Voting Group, whom we refer to as the Essex Members, collectively have the right to designate up to three individuals to be included in the slate of nominees recommended by our Board. Smith & Nephew, Inc. and Smith & Nephew (Europe) B.V., as the other members of the Voting Group, whom we refer to as the SN Members, collectively have the right to designate up to two individuals to be included in the slate of nominees recommended by our Board. The number of individuals that the Essex Members and SN Members may designate decreases if they dispose of certain percentages of the shares of our Class A common stock or Class B common stock, as applicable, that they owned as of the date of the IPO. At such time as the Essex or SN Members each own less than 10% of the shares of Class A common stock and Class B common stock that they owned as of the date of the IPO, the Essex Members or SN Members, as the case may be, will no longer have designation rights under the Stockholders Agreement. Until such time, or if the Stockholders Agreement is otherwise terminated in accordance with its terms, the parties to the Stockholders Agreement agree to vote their shares of Class A common stock and Class B common stock in favor of the election of the nominees of the Essex and SN Members to our Board upon their nomination by the nominating and corporate governance committee of our Board.
Voting Group Approvals
Under the Stockholders Agreement, any increase or decrease in the size of our Board or any committee, and any amendment to our organizational documents, in each case require the approval of the Essex and SN Members, for so long as each collectively own at least 10% of the total shares of our common stock owned by them as of the date of our IPO as follows:
Essex Members - Class A common stock
SN Members - Class A and B common stock
There were no payments made by the Company pursuant to this agreement since January 1, 2020.
Registration Rights Agreement
We entered into a registration rights agreement (Registration Rights Agreement) with the Original LLC Owners on February 16, 2021 in connection with our IPO. The Registration Rights Agreement provides the Original LLC Owners certain registration rights whereby, at the expiration of the IPO lock-up period, which will occur on August 9, 2021, the Continuing LLC Owner can require us to register under the Securities Act shares of Class A common stock issuable to it upon, at our election, redemption or exchange of its LLC Interests, and the Former LLC Owners can require us to register under the Securities Act the shares of Class A common stock issued to them in connection with certain transactions completed in connection with the IPO. The Registration Rights Agreement provides for piggyback registration rights for the Original LLC Owners. There were no payments made by the Company pursuant to this agreement since January 1, 2020.
Indemnification Agreements
We have entered into separate indemnification agreements with our directors and executive officers. The indemnification agreements provide for indemnification of our directors and executive officers for expenses, judgments, fines and settlement amounts incurred by this person in any action or proceeding arising out of this person’s services as a director or executive officer or at our request. There were no payments made by the Company pursuant to these agreements since January 1, 2020.
Director independence
Our Board undertook a review of the independence of our directors and considered whether any director has a material relationship with us that could compromise that director’s ability to exercise independent judgment in carrying out that director’s responsibilities. Our Board has affirmatively determined that Mr. Hawkins, Mr. Cowdy, Mr. Neels, Mr. Nohra, Mr. Parker, Mr. Sutter and Ms. Stalnecker are each an “independent director,” as defined under the rules of Nasdaq. In making these determinations, our Board considered the current and prior relationships that each director has with our Company and all other facts and circumstances our Board deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each director, and the transactions involving them described in this section.
Item 14.Principal Accounting Fees and Services.
The following table summarizes fees paid or accrued to our independent registered public accounting firm, Grant Thornton LLP (GT), in connection with various services for each ofinformation required by this Item is incorporated by reference from the last two fiscal years.
| | | | | | | | | | | |
| 2020 | | 2019 |
Audit Fees(1) | $ | 1,081 | | | $ | 592 | |
Audit-Related Fees(2) | 614 | | | — | |
Tax Fees(3) | — | | | — | |
All Other Fees(4) | 2 | | | 21 | |
(1)Audit fees are for services related to the annual audit of the Company’s consolidated financial statements, including the interim reviews of the Company’s quarterly financial statements, statutory audits of the Company’s foreign subsidiaries and consultations on accounting matters.
(2)Audit-related fees are related to audits of the Company’s employee benefit plans and due diligence services in connection with acquisitions.
(3)Tax fees are for domestic and international tax compliance and advisory services.
(4)All other fees are primarily for subscriptions to online research tools and training courses for professional qualifications.
Pre-approval policy and procedures
The Audit and Risk Committee has adopted a policy (Pre-Approval Policy) that sets forth the procedures pursuant to which audit and permissible non-audit services proposed to be provided by GT may be pre-approved. The Pre-Approval Policy generally provides that we will not engage GT to render any audit, audit-related, tax or other service unless the service is either (i) explicitly approved by the Audit and Risk Committee (specific pre-approval) or (ii) entered into pursuant to the pre-approval policies and procedures described in the Pre-Approval Policy (general pre-approval). Unless a type of service has received general pre-approval, it will require specific pre-approval by the Audit and Risk Committee, or by a designated membersection captioned “Report of the Audit and Risk Committee to whomof the committee has delegated the authority to grant pre-approvals, if it isBoard of Directors” contained in our definitive proxy statement for our 2024 Annual Meeting of Stockholders to be provided by GT. Any proposed services exceeding pre-approved cost levels or budgeted amounts will also require specific pre-approval.
Pre-approval fee levels or budgeted amounts for all services to be provided by GT are established annually byfiled with the Audit and Risk Committee. Any proposed services exceeding these levels or amounts require specific pre-approval bySEC within 120 days after the Audit Committee. For eachend of the fiscal year the Audit and Risk Committee may determine the appropriate ratio between the total amount of fees for audit, audit-related and tax services, and the total amount of fees for services classified as all other services.ended December 31, 2023.
Other than with respect to the annual audit of the Company’s consolidated financial statements, the chairperson of the Audit and Risk Committee is delegated the authority to pre-approve other audit services and all other services.
The Audit and Risk Committee monitors the performance of all services provided by GT and determines whether such services are in compliance with this Pre-Approval Policy.
PART IV
Item 15.Exhibits and Financial Statement SchedulesSchedules.
(a)Financial Statements. See the table of contents under Part II, Item 8. Financial Statements and Supplementary Dataof this Annual Report on Form 10-K above for the list of financial statements filed as part of this report.
(b)Exhibits.The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
| | Exhibit no. | Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewithin | Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewith |
| | | | | | | | | | | | | | |
| 2.1 | |
| 2.1 | |
| 2.1 | |
| | 2.2 | |
| | 2.2 | |
| | 2.2 | |
| | 2.3 | |
| | 2.3 | |
| | 2.3 | |
| | 3.1 | 3.1 | | | | 8-K | | 001-37844 | | 3.1 | | 2/17/2021 | |
| | | | | |
| 3.1 | |
| | 3.1 | |
| | |
| | |
| | |
| 3.2 | |
| 3.2 | |
| 3.2 | 3.2 | | | | 8-K | | 001-37844 | | 3.2 | | 2/17/2021 | |
| | | | | |
| | |
| | |
| 4.1 | |
| 4.1 | |
| 4.1 | 4.1 | | | | S-1 | | 333-252238 | | 4.1 | | 1/20/2021 | |
| | | | | |
| | |
| | |
| 4.2 | |
| 4.2 | |
| 4.2 | 4.2 | | | | * |
| | | | | |
| 10.1 | 10.1 | | | | 8-K | | 001-37844 | | 10.2 | | 2/17/2021 | |
| | | | | |
| 10.1 | |
| | 10.1 | |
| | |
| | |
| | |
| 10.2 | |
| 10.2 | |
| 10.2 | 10.2 | | | | 8-K | | 001-37844 | | 10.3 | | 2/17/2021 | |
| | | | | |
| | |
| | |
| 10.3 | |
| 10.3 | |
| 10.3 | 10.3 | | | | 8-K | | 001-37844 | | 10.1 | | 2/17/2021 | |
| | | | | |
| | |
| | |
| 10.4 | |
| 10.4 | |
| 10.4 | 10.4 | | | | 8-K | | 001-37844 | | 10.4 | | 2/17/2021 | |
| | | | | |
| | |
| | |
| 10.5† | |
| 10.5† | |
| 10.5† | 10.5† | | | | S-1 | | 333-252238 | | 10.5 | | 1/20/2021 | |
| | | | | |
| | |
| | |
| 10.6† | |
| 10.6† | |
| 10.6† | 10.6† | | | | S-1 | | 333-252238 | | 10.6 | | 1/20/2021 | |
| | | | | |
| 10.7† | | | | S-1 | | 333-252238 | | 10.7 | | 1/20/2021 | |
| | | | | |
| 10.7(a)† | | | | S-1 | | 333-252238 | | 10.7(a) | | 1/20/2021 | |
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewithin |
| 10.7(b)† | | | | S-1/A | | 333-252238 | | 10.7(b) | | 2/4/2021 | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | |
| 10.11† | | | | S-1 | | 333-252238 | | 10.9 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.9 | | | | S-1 | | 333-252238 | | 10.10 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.10 | | | | S-1 | | 333-252238 | | 10.11 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.11# | | | | S-1/A | | 333-252238 | | 10.12 | | 2/4/2021 | | |
| | | | | | | | | | | | |
| 10.12# | | | | S-1 | | 333-252238 | | 10.13 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.13# | | | | S-1 | | 333-252238 | | 10.14 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.14# | | | | S-1 | | 333-252238 | | 10.15 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.15# | | | | S-1 | | 333-252238 | | 10.16 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.16# | | | | S-1 | | 333-252238 | | 10.17 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.17# | | | | S-1 | | 333-252238 | | 10.18 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.18# | | | | S-1 | | 333-252238 | | 10.19 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewith |
| 10.7† | | | | S-1 | | 333-252238 | | 10.7 | | 1/20/2021 | | |
| | | | | | | | | | | | | |
| 10.7(a)† | | | | S-1 | | 333-252238 | | 10.7(a) | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.7(b)† | | | | S-1/A | | 333-252238 | | 10.7(b) | | 2/4/2021 | | |
| | | | | | | | | | | | |
| 10.8 | | | | S-1 | | 001-37844 | | 10.11 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.8(a) | | | | 10-Q | | 001-37844 | | 10.1 | | 11/10/2021 | | |
| | | | | | | | | | | | |
| 10.8(b) | | Amendment No. 2 to Credit and Guaranty Agreement, dated as of October 29, 2021, by and among Bioventus LLC, Oyster Merger Sub I, LLC, Oyster Merger Sub II, LLC, Misonix, Inc., certain Guarantor Subsidiaries party thereto, Wells Fargo Bank, National Association, as administrative agent and the lenders and other financial institutions party thereto. | | 8-K | | 001-37844 | | 10.1 | | 10/29/2021 | | |
| | | | | | | | | | | | |
| 10.8(c) | | | | 8-K | | 001-37844 | | 10.1 | | 7/12/2022 | | |
| | | | | | | | | | | | |
| 10.8(d) | | | | 10-K | | 001-37844 | | 10.8(d) | | 3/31/2023 | | |
| | | | | | | | | | | | |
| 10.8(e) | | | | 8-K | | 001-37844 | | 10.1 | | 1/19/2024 | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewith |
| 10.9^ | | | | S-1 | | 333-252238 | | 10.33 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.10^ | | | | S-1 | | 333-252238 | | 10.38 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.11^ | | | | S-1 | | 333-252238 | | 10.42 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.12^ | | | | S-1 | | 333-252238 | | 10.43 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.13^ | | | | S-1/A | | 333-252238 | | 10.44 | | 2/4/2021 | | |
| | | | | | | | | | | | |
| 10.14^ | | | | 10-Q | | 001-37844 | | 10.3 | | 8/12/2022 | | |
| | | | | | | | | | | | |
| 10.15^ | | | | S-1/A | | 333-252238 | | 10.47 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.16^ | | | | S-1/A | | 333-252238 | | 10.48 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.17^ | | | | S-1/A | | 333-252238 | | 10.51 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.18^ | | | | S-1/A | | 333-252238 | | 10.52 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.19^ | | | | S-1/A | | 333-252238 | | 10.55 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.20^ | | | | S-1/A | | 333-252238 | | 10.46 | | 2/4/2021 | | |
| | | | | | | | | | | | | |
| 10.21 | | | | 8-K | | 001-37844 | | 10.1 | | 11/22/2021 | | |
| | | | | | | | | | | | |
| 10.22^ | | | | 8-K | | 001-37844 | | 10.1 | | 2/28/2022 | | |
| | | | | | | | | | | | |
| 10.23^ | | | | S-8 | | 333-264050 | | 99.1 | | 4/1/2022 | | |
| | | | | | | | | | | | |
| 10.24^ | | | | S-8 | | 333-264050 | | 99.2 | | 4/1/2022 | | |
| | | | | | | | | | | | |
| 10.25 | | | | S-1 | | 333-252238 | | 10.10 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.25(a) | | | | 8-K | | 001-37844 | | 10.1 | | 6/22/2022 | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exhibit no. | | Description | | Form | | Form | File No. | | Exhibit | | Exhibit | Filing Date | | Filed / Furnished HerewithinHerewith |
10.19#10.26^ | | | | 10-Q | | 001-37844 | | 10.3 | | 5/16/2023 | | |
| | | | | | | | | | | | |
| 10.27† | | | | S-1 | | 333-252238 | | 10.9 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.28^ | | | | 10-Q | | 001-37844 | | 10.1 | | 5/16/2023 | | |
| | | | | | | | | | | | |
| 10.29^ | | | | 8-K | | 001-37844 | | 10.1 | | 4/5/2023 | | |
| | | | | | | | | | | | |
| 10.30^ | | | | 8-K/A | | 001-37844 | | 10.1 | | 4/11/2023 | | |
| | | | | | | | | | | | |
| 10.31^ | | | | 8-K | | 001-37844 | | 10.1 | | 6/9/2023 | | |
| | | | | | | | | | | | |
| 10.32^ | | | | 8-K | | S-1001-37844 | | 10.2 | | 333-2522386/9/2023 | | | 10.20 | 1/20/2021 | | |
| | | | | | | | | | | | |
10.20#10.33^ | | | | S-1 | | 333-252238 | | 10.21 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.21# | | | | S-1 | | 333-252238 | | 10.22 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.22# | | | | S-1 | | 333-252238 | | 10.23 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.23# | | | | S-1 | | 333-252238 | | 10.24 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.24# | | | | S-1 | | 333-252238 | | 10.25 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.25# | | | | S-1 | | 333-252238 | | 10.26 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.26# | | | | S-1 | | 333-252238 | | 10.27 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.27# | | | | S-1 | | 333-252238 | | 10.28 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.28# | | | | S-1 | | 333-252238 | | 10.29 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.29# | | | | S-1 | | 333-252238 | | 10.30 | | 1/20/2021 | | |
| | | | | | | | | | | | |
10.30# | | | | 8-K | | S-1001-37844 | | 10.1 | | 333-25223812/21/2023 | | | 10.33 | 1/20/2021 | | |
| | | | | | | | | | | | |
10.31#10.34^ | | | | S-1 | | 333-252238 | | 10.35 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewithin |
| 10.32# | | | | S-1 | | 333-252238 | | 10.38 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.33# | | | | S-1 | | 333-252238 | | 10.41 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.34# | | | | S-1 | | 333-252238 | | 10.42 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.35 | | | | S-1 | | 333-252238 | | 10.43 | | 1/20/2021 | | |
| | | | | | | | | | | | |
| 10.36# | | | | S-1/A | | 333-2522 | | 10.44 | | 2/4/2021 | | |
| | | | | | | | | | | | |
| 10.37# | | | | S-1/A | | 333-2522 | | 10.45 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.38# | | | | S-1/A | | 333-2522 | | 10.47 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.39# | | | | S-1/A | | 333-2522 | | 10.48 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.40 | | | | S-1/A | | 333-2522 | | 10.50 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.41# | | | | S-1/A | | 333-2522 | | 10.51 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.42# | | | | S-1/A | | 333-2522 | | 10.52 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.43# | | | | S-1/A | | 333-2522 | | 10.53 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.44# | | | | S-1/A | | 333-2522 | | 10.54 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.45# | | | | S-1/A | | 333-2522 | | 10.55 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.46# | | | | S-1/A | | 333-2522 | | 10.56 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| 10.47# | | | | S-1/A | | 333-2522 | | 10.57 | | 2/10/2021 | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewithin |
10.48# | | | | 8-K | | S-1/A001-37844 | | 10.2 | | 333-252212/21/2023 | | | 10.46 | 2/4/2021 | | |
| | | | | | | | | | | | |
10.4910.35^ | | | | | | | | | | | | * |
| | | | | | | | | | | | |
21.1 | | | | 8-K | | 001-37844 | | 10.3 | | 12/21/2023 | | |
| | | | | | | | | | | | |
| 21.1 | | | | | | | | | | | | * |
| | | | | | | | | | | | |
| | | | | | | | | | | | * |
| | | | | | | | | | | | | |
23.2 | | | | | | | | | | | | * |
| | | | | | | | | | | | |
23.3 | | | | | | | | | | | | * |
| | | | | | | | | | | | |
31.1 | | | | | | | | | | | | * |
| | | | | | | | | | | | |
| | | | | | | | | | | | * |
| | | | | | | | | | | | |
| | | | | | | | | | | | ** |
| | | | | | | | | | | | |
| 97.1 | | | | | | | | | | | | * |
| | | | | | | | | | | | |
| | | | | | | | | | | | * |
| | | | | ** | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exhibit no. | | Description | | Form | | File No. | | Exhibit | | Filing Date | | Filed / Furnished Herewith |
| 101.INS | | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | | | | | | | | | | *** |
| | | | | | | | | | | | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document | | | | | | | | | | *** |
| | | | | | | | | | | | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | | | | | *** |
| | | | | | | | | | | | |
| 101.DEF | | Inline XBRL Extension Definition Linkbase Document | | | | | | | | | | *** |
| | | | | | | | | | | | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document | | | | | | | | | | *** |
| | | | | | | | | | | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | | | | | | | | | *** |
| | | | | | | | | | | | |
| 104 | | Cover Page Interactive Data File - the cover page XBRL tags are embedded within the Inline XBRL document contained in Exhibit 10 | | | | | | | | | | *** |
* Filed herewith
** Furnished herewith
# Indicates management contract or compensatory plan.*** Submitted electronically herewith
† Certain portions of this exhibit have been omitted pursuant to Regulation S-K, Item 601(b)(601)(b)(10)(iv).
^ Indicates management contract or compensatory plan.
(c)Financial Statement Schedules. Schedules for which provision is made in the applicable accounting regulation of the Securities and Exchange Commission have been omitted because they are not applicable, not required or the information required is given in the Consolidated Financial Statements and notes thereto set forth above underPart II, Item 8. Financial Statements and Supplemental Data.
Item 16.Form 10-K Summary.
None
SIGNATURESSIGNATURE
Pursuant to the requirements of Section 13 or 15(d) of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| | BIOVENTUS INC. |
| | |
| By: | Bioventus Inc./s/ | Robert E. Claypoole |
| | Name: | | | Robert E. Claypoole |
| | Title: | By: | /s/ | Kenneth M. Reali |
| | Name: | Kenneth M. Reali |
| | Title: | President, Chief Executive Officer and Director (Principal Executive Officer) |
| March 12, 2024 | | | |
March 26, 2021
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the Registrantregistrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Name | | Date | | Date | Title |
| | | | |
/s/ Kenneth M. RealiRobert E. Claypoole | | March 12, 2024 | | March 26, 2021 | President, Chief Executive Officer and Director
(Principal Executive Officer) |
Kenneth M. RealiRobert E. Claypoole | | | |
| | | | |
| | | | |
/s/ Gregory O. AnglumMark L. Singleton | | March 12, 2024 | | March 26, 2021 | Senior Vice President and Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer) |
Gregory O. AnglumMark L. Singleton | | | |
| | | | |
| /s/ William A. Hawkins III | | March 12, 2024 | | March 26, 2021 | Chairman |
| William A. Hawkins III | | | | |
| | | | |
| /s/ John A. Bartholdson | | March 12, 2024 | | Director |
| John A. Bartholdson | | | | |
| | | | |
| /s/ Patrick J. Beyer | | March 12, 2024 | | Director |
| Patrick J. Beyer | | | | |
| | | | |
| /s/ Philip G. Cowdy | | March 12, 2024 | | March 26, 2021 | Director |
| Philip G. Cowdy | | | | |
| | | | |
| /s/ Mary Kay Ladone | | March 12, 2024 | | Director |
| Mary Kay Ladone | | | | |
| | | | |
| /s/ Michelle McMurry-Heath | | March 12, 2024 | | Director |
| Michelle McMurry-Heath | | | | |
| | | | |
| /s/ Guido J. Neels | | March 12, 2024 | | March 26, 2021 | Director |
| Guido J. Neels | | | | |
| | | | |
| /s/ Guy P. Nohra | | March 12, 2024 | | March 26, 2021 | Director |
| Guy P. Nohra | | | | |
| | | | |
/s/ David J. Parker | | March 26, 2021 | | Director |
David J. Parker | | | | |
| | | | |
| /s/ Susan M. Stalnecker | | March 12, 2024 | | March 26, 2021 | Director |
| Susan M. Stalnecker | | | | |
| | | | |
| /s/ Martin P. Sutter | | March 12, 2024 | | March 26, 2021 | Director |
| Martin P. Sutter | | | | |
| | | | |